Abstract
Introduction
Multiple myeloma (MM)remains an incurable disease because most of the available drugs do not destroy clonogenic tumor cell (CTC). Natural Killer (NK) cells exert cytotoxicity against MM cells; improving NK cell cytotoxicity might be part of the mechanism of action of effective anti-myeloma drugs such as lenalidomide or bortezomib. By coculture with the genetically-modified K562-mb15-41BBL cell line it is possible to expand ex vivo large numbers of activated NK cells from MM patients. We are conducting a phase I clinical trial to evaluate feasibility, safety and tolerability of these NK cells (termed NKAEs) infused in MM patients an autologous setting (EudraCT 2012-000514-11). Because the activity of NKAEs against MM CTCs is unknown, we addressed this issue and analyzed NK cell ligands and receptors pathways mediating CTCs destruction.
Methods and Patients
Peripheral blood (PB) was collected from MM patients (n=36) or healthy donors (n=14).To activate and expand NK cell from MM patients, peripheral blood mononuclear cell were co-cultured with K562-mb15-41BBL cells and 100 IU/ml IL-2.
We used time-resolved fluorescence to detect activity of NK cells on bulk MM cells and methylcellulose clonogenic assays to determine NK cell specific activity on MM CTCs. We analyzed NK and MM receptor expression profile by flow cytometry and Real Time PCR, and identified the side population (SP) by DyeCycle Violet efflux. Three MM patients on 2nd or later relapse have been enrolled in the phase I clinical trial to date. We collected 200 ml of PB from patients to produce autologous NKAEs under GMP conditions and cells were harvested on day 14 and 21 for infusions. Four cycles of pharmacological treatment with 2 infusions of 7.5 x 106 autologous NKAEs/kg on day 1 and 8 of each cycle were performed.
Results
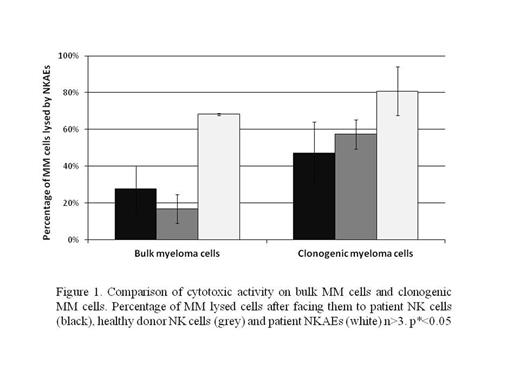
NK cells from patients (n=20) produced 26.6±12.7% lysis of bulk MM cells, similar to NK cells from healthy donors (17±7.8%), while cytotoxicity by NKAEs from MM patients was 68± 0.7% (n=3) at 8:1 ratio. In methylcellulose assays, MM cell killing was higher on CTCs (47±16.8% in MM patients and 57±8% in healthy donors) than on corresponding bulk MM cells (p<0.01), with a maximum effect at 32:1 (58±22% and 87.5±6.5%, respectively). In contrast, killing of CTCs with patient NKAEs (n=6) was 81±13% (8:1) (figure 1), with a strong dose-dependent relationship (maximum effect 95.1±6% at 32:1). NKAEs (n=5) showed over-expression of NKG2D and NKp30 receptors compared to NK cells (n=18). Blocking NKAEs NKp30 or NKG2D prior to methylcellulose assay (n=5) caused a significant increase in colony growth. Flow cytometric analysis of MM cells demonstrated that the SP cells have same expression profile of NKG2D ligands when compared to non-SP cells in 7 MM cell lines. Nevertheless, they showed down-regulation of apoptosis receptors and expression of DNAM-1 ligands. The NKp30 ligand B7H6 was downregulated in both MM cell lines and bone marrow MM cells.
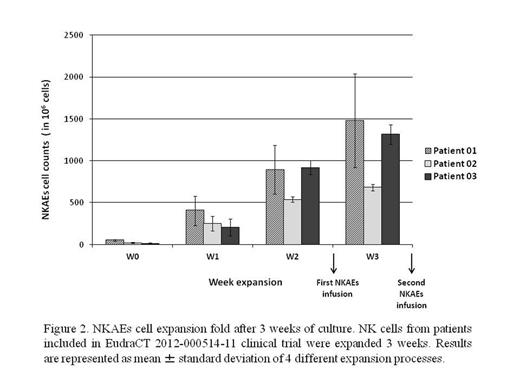
Two MM patients undergoing lenalidomide treatment and 1 MM patient who received bortezomib and bendamustine treatment, all with persistent or progressing disease, have been enrolled in the clinical trial and received a total of 20 NKAEs infusions. We observed grade II and III neutropenia, which did not require dose adjustment. MM patients had 15% (±5%) NK cells of PB mononuclear cells. We collected 30 x 106 (±17 x 106) NK cells from patients PB. After 1 week NKAEs number increased x9 fold, at 2nd week fold was x29. We collected 550 x 106 (±50x 106) NKAEs from culture for the first infusion. At 3rd week NKAEs number reached 1077 x 106 and 87.5% (±11.5%) purity of NKAEs (figure 2). All patients are still alive after one 1 year of starting the treatment. One patient achieved a partial response and maintained it for 13 months after NKAEs infusions. Another patient, who started NKAEs infusion while in relapse, achieved stable disease and maintained it for 11 months, after which disease progressed. The third patient progressed two months after stopping the treatment for unrelated toxicity.
Conclusion
NKAEs from MM patients have enhanced cytotoxicity against MM CTCs, which is mediated through NKG2D receptor and their cognate ligands.
Clinical grade NKAEs can be obtained from MM patients even during treatment, and multiple infusions of NKAEs are feasible without notable toxicities. These results and clinical observations warrant further development of NKAEs infusion as a treatment modality for refractory MM.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.