Abstract
Background: Peripheral T-cell lymphoma (PTCL) is a heterogeneous group of non-Hodgkin lymphomas associated with a poor prognosis for most subtypes. Anthracycline-based therapies such as cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) are commonly used for frontline treatment of PTCL; however, durable remissions are uncommon with these regimens. Patients with relapsed or refractory (R/R) PTCL and platelet counts <100,000/µL typically have poorer outcomes, and often are not eligible to participate in clinical trials or require dose reductions to safely administer many of the currently approved agents in this setting. Thus, it is important to identify appropriate treatment strategies for these patients, particularly in the salvage setting.
Belinostat (Beleodaq) is a potent, pan-histone deacetylase inhibitor that was recently approved in the United States for the treatment of patients with R/R PTCL. Approval was based on results from the pivotal Phase 2 BELIEF study of belinostat in R/R PTCL (N = 129 enrolled, N = 120 evaluable), which demonstrated durable clinical benefit (objective response rate [ORR] 25.8%) and tolerability. This analysis presents BELIEF study data for the subgroup of patients who had thrombocytopenia (<100,000/µL) at study entry (n = 24 enrolled, n = 20 evaluable).
Methods: Patients with R/R PTCL received belinostat as a 1000 mg/m2 intravenous infusion on Days 1-5 of 21-day cycles. The primary endpoint of the study was ORR (complete response [CR] + partial response [PR]) determined by an Independent Review Committee. For this subset analysis, efficacy and safety data for the 20 evaluable patients with low baseline platelet counts were examined and compared to the overall study population.
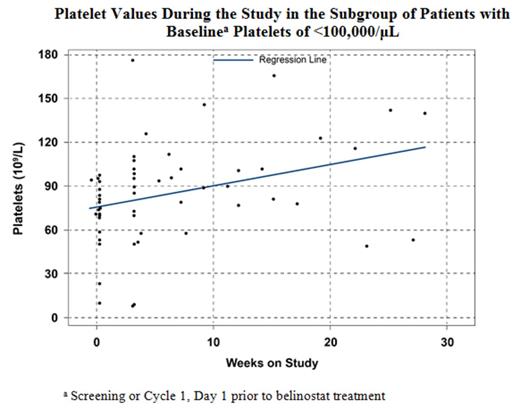
Results : For both the low baseline platelet count (<100,000/µL) subgroup (n = 20 evaluable) and the overall evaluable study population, a median of 2.0 treatment cycles and 10.0 belinostat doses were administered, with a relative dose intensity of 98.5% and 98.3%, respectively. Baseline bone marrow involvement was present for 50% of patients with low baseline platelet counts and 29% of the overall population. For 2/20 patients, the belinostat dose was reduced for low platelets. The ORR in the low baseline platelet count subgroup was 15%, with 2 PRs and 1 CR. The median duration of response by International Working Group criteria was 4.1 months, with a median overall survival of 4.3 months and median progression-free survival of 1.3 months, based on a median follow-up of 11.2 months (Table). The majority of Grade ≥3 treatment-related adverse events (AEs) reported in >5% of the evaluable patients with low baseline platelet counts were largely hematologic, including anemia (5.4% overall and 10.0% low platelet group), thrombocytopenia (4.7% and 15.0%), leukopenia (2.3% and 10.0%), and neutropenia (4.7% and 10.0%). Platelet counts tended to increase over time on study for the low baseline platelet subgroup (Figure).
Conclusions: Complete and partial responses were seen with belinostat overall and in the subgroup of patients with low baseline platelet counts. Patients with baseline platelet counts <100,000/µL tolerated belinostat at a high dose intensity that was similar to that of patients with baseline platelet counts ≥100,000/µL, did not experience a higher proportion of AEs, and benefited from belinostat treatment. Thus, belinostat is suitable for use in patients with R/R PTCL and low baseline platelet counts, and may also be useful in developing new treatment regimens in combination with other cytotoxic agents.
Summary of IRC Assessment of Efficacy Endpoints by Baseline Platelet Count
| E ndpoint . | All Evaluable Patients (N = 120) . | Platelets ≥100,000/µL (N = 100) . | Platelets <100,000/µL (N = 20) . |
|---|---|---|---|
| ORR (CPRG), n (%) | 31 (25.8) | 28 (28.0) | 3 (15.0) |
| Median DoR, months (95% CI) | 13.6 (4.5-29.4) | 13.6 (5.6-29.4) | 4.1 (2.2-9.8) |
| Median PFS, months (95% CI) | 1.6 (1.4-2.7) | 1.8 (1.5-2.8) | 1.3 (1.1-1.5) |
| Median OS, months (95% CI) | 7.9 (6.1-13.9) | 9.2 (6.4-17.7) | 4.3 (2.4-7.9) |
| Median TTR, weeks (95% CI) | 5.6 (4.3-50.4) | 5.6 (4.3-50.4) | 6.4 (4.3-12.7) |
| E ndpoint . | All Evaluable Patients (N = 120) . | Platelets ≥100,000/µL (N = 100) . | Platelets <100,000/µL (N = 20) . |
|---|---|---|---|
| ORR (CPRG), n (%) | 31 (25.8) | 28 (28.0) | 3 (15.0) |
| Median DoR, months (95% CI) | 13.6 (4.5-29.4) | 13.6 (5.6-29.4) | 4.1 (2.2-9.8) |
| Median PFS, months (95% CI) | 1.6 (1.4-2.7) | 1.8 (1.5-2.8) | 1.3 (1.1-1.5) |
| Median OS, months (95% CI) | 7.9 (6.1-13.9) | 9.2 (6.4-17.7) | 4.3 (2.4-7.9) |
| Median TTR, weeks (95% CI) | 5.6 (4.3-50.4) | 5.6 (4.3-50.4) | 6.4 (4.3-12.7) |
Abbreviations: CI = confidence interval; CPRG = Central Pathology Review Group; DoR = duration of response; IRC = Independent Review Committee; ORR = objective response rate; OS = overall survival; PFS = progression-free survival; TTR = time to response
Horwitz:Celgene: Consultancy, Research Funding; Millenium: Consultancy, Research Funding; Infinity: Research Funding; Kiowa-Kirin: Research Funding; Seattle Genetics: Consultancy, Research Funding; Spectrum: Consultancy, Research Funding; Amgen: Consultancy; Bristol-Myers Squibb: Consultancy; Jannsen: Consultancy. Bhat:Spectrum Pharmaceuticals: Employment. Choi:Spectrum Pharmaceuticals: Employment. Allen:Spectrum Pharmaceuticals: Employment.
Author notes
Asterisk with author names denotes non-ASH members.