Abstract
Introduction: PI3K-delta signaling is critical for activation, proliferation and survival of B cells, and is hyperactive in many B-cell malignancies. Idelalisib, a selective oral inhibitor of PI3Kd, demonstrated considerable clinical activity as monotherapy in recurrent (Flinn, Blood 2014) or refractory iNHL subjects (Gopal, NEJM 2014). FDA granted accelerated approval for Idelalisib (ZYDELIG®) in patients who have received at least two prior systemic therapies with relapsed FL or SLL. This study evaluated Idelalisib in combination with rituximab, bendamustine, or both. We now present mature safety and response data with up to 4 years of follow up.
Methods: Eligible patients had relapsed/refractory indolent NHL. Idelalisib (Z) was administered continuously with rituximab (R) (375 mg/m2 given weekly for 8 doses) (R/Z regimen), with bendamustine (B) (90 mg/m2 given on Days 1 and 2, for 6 cycles) (B/Z regimen), or in combination with R (375 mg/m2, on Day 1) and B (90 mg/m2 given on Days 1 and 2 of each cycle, for 6 cycles (BR/Z regimen). Initial subjects in the R/Z and B/Z groups (n=8 each), received Idelalisib 100 mg/dose BID. Thereafter, all patients received an Idelalisib dose of 150 mg/dose BID. Tumor response was evaluated according to standard criteria (Cheson 2007). The cutoff date for this analysis was June 2014, 26 months after the last patient enrolled.
Results: Between April 2010 and May 2012, 79 subjects with iNHL were enrolled (including 59 with FL, 15 with SLL, and 5 with MZL). Median [range] age was 61 [37-84] years. At baseline patients had elevated beta-2 microglobulin (59%), stage IV disease (58%), bulky adenopathy (> 5cm) (48%), anemia (Hgb <12gm/dL) (41%), and elevated LDH (28%). Patients had a median number of 3 prior therapies (range 1 -11). Most patients had received a rituximab-containing regimen (98%), an alkylating agent (86%), or an anthracycline (53%). Approximately 46% of patients were refractory to their last pre-study therapy and 58% of patients were refractory to rituximab.
Frequent adverse events (all grade %/grade 3-4 %) included pyrexia (54/3), nausea (44/0), fatigue (43/4), diarrhea (39/15), rash (38/9), cough (35/0), pneumonia (22/19), pneumonitis (4/3), and febrile neutropenia (3/3). Laboratory abnormalities included lymphopenia (75/62), neutropenia (56/41), anemia (47/10), thrombocytopenia (42/8), and serum transaminase elevations (56/17).
Drug was temporarily held for Grade 3/4 ALT/AST elevations, and 8/13 pts (62%) were re-treated without recurrence of ALT/AST elevation. 27% of pts have discontinued therapy due to adverse events.
Of the 79 subjects enrolled, 64 had an objective response with an ORR of 81% (95% CI: 70.6-89.0). Complete responses were demonstrated in 26 patients (33%), and partial responses in 38 patients (48%). In addition, 7 patients had stable disease (9%), and 4 patients had progressive disease (5%) as best response on-study. Four patients were non-evaluable, as they did not have follow up CT scans. By treatment subgroup, the ORR were (n=24/32) 75% (95% CI: 57-89) for R/Z, (n=29/33) 88% (95% CI: 72-97) for B/Z, and (n=11/14) 79% (95% CI: 49-95) BR/Z. The CR rates were 25% (n=8/32), 36% (n=12/33), and 43% (n=6/14) respectively; stable disease was noted in 4/32 patients (13%), 3/33 patients (9%), and 0/14 patients in the three groups respectively. ORR/CR by iNHL subtype is: FL (81%/39%), SLL (73%/13%), and MZL (100%/20%).
The median progression-free survival is 32.8 months. Median PFS for R/Z group is 29.7 months, B/Z group 32.8 months, and BR/Z group 37.1 months. The PFS at 24 months was 55%, 64%, and 71% for the R/Z, B/Z, and BR/Z groups respectively.
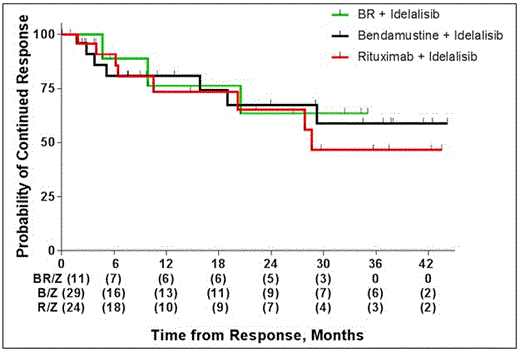
The median duration of response has not yet been reached. Median DOR for the R/Z group is 28.6 months, for the B/Z, and BR/Z groups it is not yet reached. The DOR at 24 months was 65%, 67%, and 64% for the R/Z, B/Z, and BR/Z groups respectively. Figure 1: Median overall survival is not yet reached.
Conclusions: Idelalisib in combination therapy was well tolerated, had an acceptable safety profile, and was highly effective in this recurrent iNHL population with an ORR of 81%, and CR rate of 33%. Responses are durable beyond 2 years, supporting further evaluation of these combination regimens. Phase 3 trials evaluating the efficacy of Idelalisib in combination with R or BR in iNHL are ongoing (NCT01732913, NCT01732929).
de Vos:Gilead Sciences: Research Funding. Off Label Use: Zydelig is a kinase inhibitor indicated for the treatment of patients with: 1) Relapsed chronic lymphocytic leukemia (CLL), in combination with rituximab, in patients for whom rituximab alone would be considered appropriate therapy due to other co-morbidities; 2) Relapsed follicular B-cell non-Hodgkin lymphoma (FL) in patients who have received at least two prior systemic therapies; and 3) Relapsed small lymphocytic lymphoma (SLL) in patients who have received at least two prior systemic therapies.. Wagner-Johnston:Gilead Sciences: Research Funding. Coutre:Gilead Sciences: Research Funding. Flinn:Gilead Sciences: Research Funding. Schreeder:Gilead Sciences: Research Funding. Fowler:Gilead Sciences: Research Funding. Sharman:Gilead Sciences: Research Funding. Boccia:Gilead Sciences: Research Funding. Barrientos:Gilead Sciences: Research Funding. Rai:Gilead Sciences: Research Funding. Boyd:Gilead Sciences: Research Funding. Furman:Gilead Sciences: Research Funding. Holes:Gilead Sciences: Employment, Equity Ownership. Kim:Gilead Sciences: Employment, Equity Ownership. Godfrey:Gilead Sciences: Employment, Equity Ownership. Leonard:Gilead Sciences: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.