Abstract
Introduction
Acute Myeloid Leukemia (AML) is a heterogeneous disease of myeloid progenitor cells where clonal expansion of these cells overwhelms the bone marrow leading to hematopoietic failure. Interest in identifying disruption in key regulatory pathways has led to potential for novel therapies. One particular cell signaling pathway, namely that of AKT (PKB), has been shown to correlate with AML prognosis. It has been shown previously that Protein Phosphatase 2A (PP2A) is responsible for the dephosphorylation of AKT at Thr 308 leading to decreased AKT activity. PP2A is a heterotrimeric complex consisting of a catalytic (C), scaffolding (A) and one of many regulatory (B) subunits. Studies have demonstrated that the regulatory subunit, B55α, is responsible for targeting the PP2A holoenzyme to AKT to dephosphorylate Thr 308 (Kuo et al, 2008). In fact, B55α expression has been shown to be inversely correlated with AML prognosis and Thr 308 phosphorylation of AKT (Ruvolo et al, 2011). Despite this fact, no studies have specifically demonstrated a mechanism whereby B55 Alpha expression is regulated in AML. In addition, although the potential exists for targeted therapy in patients through either activation of B55α-PP2A or disruption of AKT activity, the effectiveness of these therapies in AML has yet to be defined. In the following study we demonstrate novel loss of function mutations in the B55α gene identified in 3 AML patient samples out of 12 tested, and their effect on downstream cellular signaling pathways, including AKT, showing a possible mechanism by which B55α may act as a tumor suppressor in myeloid cells.
Results
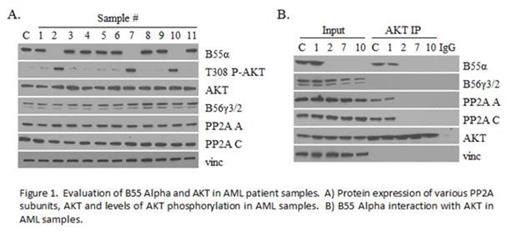
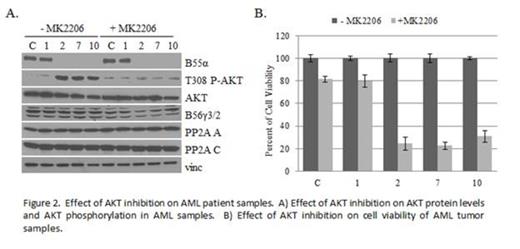
Both genomic and cDNA were analyzed from leukophoresis samples of 12 unselected patients with known diagnosis of AML whose clinical data remained unknown to the primary investigator until sequencing results were completed. Two novel B55α mutations were identified in 3 of the patient samples. In 2 samples, the same nonsense mutation was identified which led to a premature stop codon at codon 77. Interestingly both of these patients had M1 AML with FLT3 ITD positivity and NPM1 mutation positivity as well as >90% blasts on marrow smear at presentation. The other mutation was a single base pair deletion leading to a frame shift causing a premature stop codon at codon 25. This patient had residual AML NOS after partial treatment and was negative for both FLT3 and NPM1 mutations. B55α protein expression was undetectable in these 3 samples, but present in the other non-mutant samples (Figure 1A). Another regulatory B subunit, B56γ, was unaffected in all samples. Lack of B55α expression correlated with loss of B55α-PP2A complexes and loss of PP2A-AKT interaction as well as increased levels in AKT Thr308 phosphorylation (Figure 1B). Samples containing B55α mutations demonstrated enhanced sensitivity to the AKT inhibitor MK2206 (Figure 2), a compound currently undergoing evaluation as a cancer chemotherapeutic in clinical trials (Yap et al, 2011). In addition, these samples were less sensitive to the PP2A activator FTY720 (Neviani et al, 2007).
Conclusion
Novel AML-associated mutations were identified in the B55α subunit of PP2A. The mutations led to loss of B55α protein expression and loss of B55α specific PP2A complexes within the cell. Loss of B55α PP2A disrupted interaction between PP2A and AKT and led to increased phosphorylation of AKT at Thr 308, a residue important for AKT activation. It has been previously shown that decrease in B55α expression correlates with recurrence of AML, however here we demonstrate novel loss of function mutations within the B55α gene that have the significant consequence of activating a known oncogenic protein, AKT. These samples demonstrate enhanced sensitivity and responsiveness to AKT inhibition by MK2206 compared to cells with intact B55α protein. Taken together our data illustrate a significant pathway in leukemogenesis involving one specific subset of PP2A holoenzymes. In addition, our data suggest that the presence of B55α loss of function mutations may enhance tumor responsiveness to targeted therapy by AKT inhibition. Further studies are required to evaluate the clinical efficacy of such therapies in vivo, however the results indicate a novel molecular targeting approach for optimizing AML therapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.