Abstract
Introduction: Published trial data shows that ruxolitinib improves both splenomegaly-related and nonsplenomegaly-related constitutional symptoms in patients with intermediate-2 and high risk myelofibrosis (MF). However, few trial-based assessments of ruxolitinib in lower-risk MF patients have been conducted and no studies to date have made such assessments in a real-world population. In this study, we assessed changes in spleen size and constitutional symptoms during ruxolitinib treatment for lower-risk MF patients in real-world clinical settings.
Methods: This was a retrospective, observational review of anonymized medical record data collected in January 2014 by 49 hematologists and oncologists in the United States. Patient inclusion criteria were: (1) diagnosed with lower-risk MF (International Prognostic Scoring System score of 0 or 1); (2) first treated with ruxolitinib ≥3 months before the medical record abstraction date; (3) ≥18 years of age at ruxolitinib initiation; (4) complete medical history from MF diagnosis until the medical record abstraction date; and (5) never enrolled in an MF-related interventional trial. Minimum quotas of n=50 and n=25 were set for intermediate-1 and low-risk patients, respectively, with a predetermined maximum of 110 patients in the combined total. Spleen size and constitutional symptoms were retrospectively observed at MF diagnosis, at ruxolitinib initiation, and at best response while on ruxolitinib. Spleen size was captured via predefined categories of no splenomegaly (spleen not palpable), very mild or mild splenomegaly (<10 cm palpated), moderate splenomegaly (10-20 cm palpated), or severe splenomegaly (>20 cm palpated). Symptoms of interest included those captured in the MPN Symptom Assessment Form (MPN-SAF), which were categorized as mild, moderate, or severe based on medical notes recorded at each time point. For this abstract, we present findings on the 7 most commonly observed MPN-SAF symptoms in our sample. This study was exploratory and used only descriptive analyses.
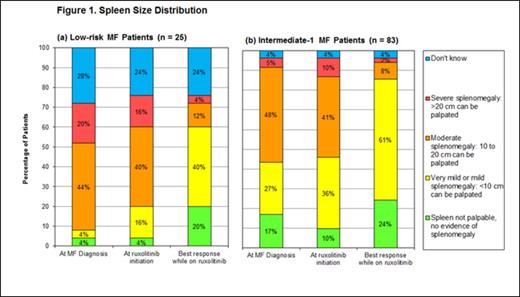
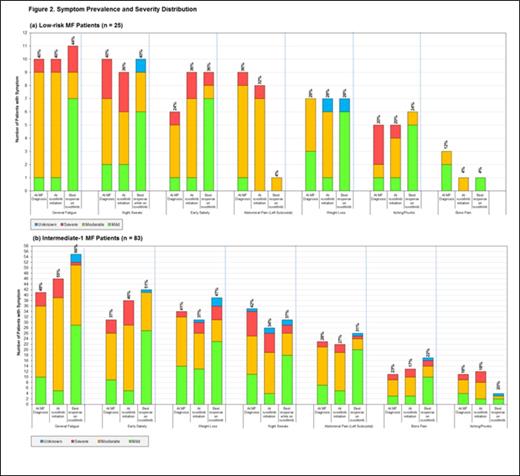
Results: A total of 108 patients were included in the study (25 low-risk and 83 intermediate-1 patients). Ruxolitinib start dates spanned January 2012 – November 2013. All low-risk patients were ≤65 years of age, and nearly 80% of intermediate-1 patients were ≤65 years of age. The majority of patients in both risk groups (60% and 69%, respectively) were male. A higher proportion of intermediate-1 patients were positive for JAK2 V617F mutation (72%) than low-risk patients (56%). Most patients (92% of low-risk, 77% of intermediate-1) were still receiving ruxolitinib treatment at the medical record abstraction date. Median observed ruxolitinib exposure time was approximately 8 months in both risk groups. In low-risk patients, the combined proportion of patients with moderate or severe splenomegaly (≥10 cm palpated spleen) decreased from 64% at MF diagnosis to 16% at best response during ruxolitinib treatment (Figure 1a). Similar findings were observed for intermediate-1 patients: the proportion with moderate or severe splenomegaly decreased from 53% at MF diagnosis to 10% at best response (Figure 1b). General fatigue was the most commonly observed constitutional symptom in both low-risk and intermediate-1 patients (Figures 2a and 2b). Shifts in symptom severity from more severe to less severe were observed in both low-risk and intermediate-1 patients. Among low-risk patients with fatigue, the proportion with moderate or severe fatigue decreased from 90% at MF diagnosis to 37% at best ruxolitinib response; in intermediate-1 patients, the decrease was from 76% at MF diagnosis to 42% at best response. For most other symptoms, similar improvements in severity distribution were observed.
Conclusions: Both low-risk and intermediate-1 MF patients experienced a substantial decrease in spleen size from MF diagnosis through ruxolitinib treatment in real-world clinical settings. Furthermore, for most symptoms examined, there was a distinct improvement in the distribution of symptom severity at the time of best response during ruxolitinib treatment. These findings suggest that lower-risk MF patients may benefit clinically from ruxolitinib treatment. Further studies are needed to assess adverse effects and evaluate the benefit-risk tradeoff of ruxolitinib.
Davis:Novartis Pharmaceuticals Corporation: Research Funding. Kaye:Novartis Pharmaceuticals Corporation: Research Funding. Cote:Novartis: Employment. Off Label Use: The study discusses use of ruxolitinib in patients with lower-risk (IPSS 0 or 1) myelofibrosis (MF) in real-world practice; currently ruxolitinib is only indicated in higher-risk MF (IPSS >1).. Gao:Novartis: Employment. Ronco:Novartis: Employment. Seifeldin:Novartis: Employment. Mendelson:Novartis: Employment.
Author notes
Asterisk with author names denotes non-ASH members.