Abstract
Background
Polycythemia vera (PV) is a clonal myeloproliferative disorder characterized by erythrocytosis, splenomegaly and a frequently burdensome symptom profile. Despite current guidelines of aspirin, phlebotomy, and selective cytoreduction, many patients have inadequately controlled PV-related symptoms and/or disease features. We performed a comparison of PV symptom burden/disease feature phenotypes to understand unmet needs in current medical management.
Methods
Data was collected prospectively amongst an international cohort of PV patients including symptom burden, demographics, and disease features. Subgroups were identified who had previously failed hydroxyurea (PV-HU), required ongoing phlebotomy (PV-P), had palpable splenomegaly (PV-S), or had all 3 features (PV-HUPS). Control groups were derived from the remaining PV patients lacking the specified subgroup trait; patients in whom the trait status was unknown were excluded from each respective control group. All participants completed the MPN specific symptom burden questionnaire (MPN-SAF TSS (MPN-10 – Table 1)) and had no prior history of splenectomy. Surveyed symptoms on the MPN-10 included the patient's perceptions of common MPN-related symptoms and overall quality of life (QOL) on a 0 (absent) to 10 (worst imaginable) scale. PV risk scores were calculated using the 2013 criteria (Leukemia 2013). Comparison of symptoms between groups employed t-tests.
Results
Patient Demographics and Disease Features Between Phenotypic Groups
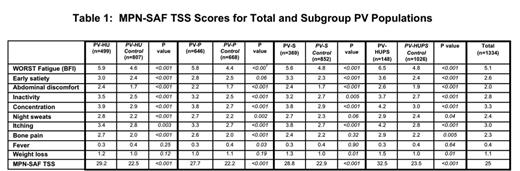
A total of 1334 PV patients completed the MPN-10, and were assigned to categories of PV-HU (499 [37%]), PV-P (646 [48%]), PV-S (369, [28%]), and PV-HUPS (148 [12%]). The demographics between these groups were similar (comparable age (median range 60-63), PV risk scores (mean risk range: Low 16.4-23.7%; Intermediate 31.4%-36.6%; High 42.8%-47%). Mean hemoglobin was similar among PV subgroups (range 14.4-14.9); PV-HUPS had a higher mean WBC count (20.3 g/dL vs. 8.8-11.8 g/dL) and platelet count (703.5 x 10(9)/L vs. 327.5-462.8 x 10(9)/L), and disease duration (11.5 years vs. 6.4-8.8 years). Prior thrombosis was most common in PV-S patients (28.5% vs. 21.8-25.2%) and prior hemorrhage was most common in PV-HUPS patients (23.8% vs. 13.7-15.8%).
Symptom Burden
The MPN-10 scores of each problematic PV phenotype (HU, P, S, HUPS) were compared to the remainder of the PV cohort lacking the trait (PV-control; Table 1). Both individual symptom scores and TSS were highest for PV-HUPS patients (mean TSS 32.5 vs. 27.7-29.2). All problematic PV subgroups demonstrated significant differences for individual symptoms and TSS compared to PV-control. Comparing "problematic" subgroup responses, PV-HU patients described more inactivity whereas PV-S patients described more early satiety and pruritus. No statistical differences were noted in PV-HU, PV-P and PV-HUPS patient responses to MPN-10 items of "fever" and "weight loss".
Discussion
PV patients who have either failed HU, are undergoing phlebotomy and/or have splenomegaly exhibit moderate to severe symptomatology and demonstrate unmet medical need for management. As evidenced in this study, considerable overlap in symptomatology exists in PV-HU, PV-P, PV-S and PV-HUPS. Current randomized trials of JAK inhibitors have demonstrated benefits in a PV-HUPS phenotype. This data suggests that PV patients with any evidence of inadequate control (PV-HU, P, or S) have similarly unmet needs and may be candidates for clinical trials, intensification of medical therapy or perhaps JAK inhibitor therapy.
Kiladjian:Shire Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees. Zweegman:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Millennium: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees, Research Funding. Besses:Shire Pharmaceuticals: honoraria for educational lectures Other. Birgegard:Shire Pharmaceuticals: Consultancy, Honoraria, Research Funding. Etienne:Novartis, BMS, Pfizer, Ariad: Honoraria. Roy:Merck: Peg-Interferon provided for academic clinical trial in CML Other. te Boekhorst:Novartis: Consultancy. Griesshammer:Novartis: Honoraria; Shire: Honoraria; Sanofi: Honoraria; Amgen: Honoraria; Roche: Honoraria. Mesa:Incyte Corporation, CTI, NPS Pharma, Inc., Gilead Science Inc., Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.