Abstract
Introduction:
BCL-2 is an anti-apoptotic protein that is commonly overexpressed in hematologic malignancies, including Non-Hodgkin Lymphoma (NHL). ABT-199 is a selective, potent, orally bioavailable BCL-2 inhibitor. It has demonstrated antitumor activity as monotherapy in patients with NHL in a Phase Ib single agent study. Patients with indolent NHL typically recur after standard treatments (R-CHOP, R-CVP). Bendamustine (B) and rituximab (R) combination therapy has demonstrated clinical activity in relapsed or refractory (R/R) patients. Preclinical evidence suggests that ABT-199 enhances the efficacy of BR. Here we report findings from a Phase I, open-label, multicenter study of ABT-199 in combination with BR in patients with R/R NHL.
Methods:
The primary objectives were evaluation of the safety, pharmacokinetics (PK), preliminary efficacy, and maximum tolerated dose (MTD) as well as recommended Phase 2 dose (RPTD) of ABT-199 in combination with BR in patients with R/R NHL. In the dose-escalation portion of the study, patients were treated on a 28 day cycle with ABT-199 with doses from 50 to 400 mg and following 3 dosing schedules: 3 days/cycle (d/cycle), 7 d/cycle, 28 d/cycle. The BR regimen was 6 cycles of treatment: B (2 d/cycle, 90mg/m2) and R (1 d/cycle, 375mg/m2). The dosing schedule of ABT-199 and BR was staggered during Cycles 1–3 for PK analyses; ABT-199 followed by BR was administered on Day 1 of Cycles 4-6. Patients could continue ABT-199 monotherapy following completion of BR. Dose limiting toxicities (DLTs), adverse events (AEs), PK parameters, and disease responses by IWG criteria were evaluated. Response assessments occurred on day 1 of cycle 3, 5 and 7.
Results:
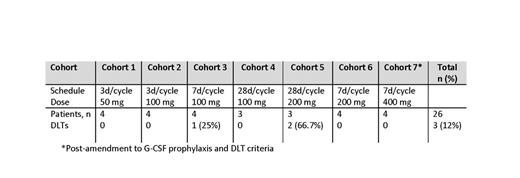
As of July 3, 2014, 26 patients have been treated with ABT-199 in combination with BR. The median age was 61.5 (range 29-80) years and 17 (65%) patients were male. Fifteen (57.7%) patients had follicular lymphoma (FL), 8 (30.8%) had diffuse large B-cell lymphoma (DLBCL), and 3 (11.5%) had marginal zone lymphoma (MZL). All were previously treated with R or R-based combination therapy. Thirteen (50%) have discontinued due to progressive disease (PD); there were no discontinuations due to AEs. Eleven (42%) had completed 6 cycles of BR treatment. The most common treatment-emergent AEs (in >25% patients) in a pooled dataset were nausea (65.4%), anemia and neutropenia (each in 42.3%), diarrhea (38.5%), thrombocytopenia (34.6%), hyperglycemia (30.8%), vomiting and hypokalemia (each in 26.9%). Grade 3/4 AEs (in >10% patients) were neutropenia (26.9%), anemia and thrombocytopenia (each in 19.2%), leukopenia (15.4%), febrile neutropenia and decreased lymphocyte count (each in 11.5%). Febrile neutropenia (11.5%) was the SAE occurring in more than 10% of patients. There were no AEs leading to death. One patient in cohort 3 (100 mg x 7d/cycle) experienced a DLT of neutropenia and 2 patients in cohort 5 (200 mg x 28 d/cycle) experienced a DLT (n=1 febrile neutropenia; n=1 thrombocytopenia). Dose frequency was decreased to 7 d/cycle for patients in cohort 6. No DLTs were observed in subsequent cohorts following an amendment to G-CSF prophylaxis and DLT criteria. Preliminary PK data suggest there is no apparent interaction between ABT-199 and B. Of 26 evaluable patients, 5 (19.2%) had a complete response (CR) and 11 (42.3%) had a partial response (PR). To date, the overall response rate (CR+PR) for ABT-199+BR is 61.5% (16/26) for all patients, 73.3% (11/15) for patients with FL, and 37.5% (3/8) for patients with DLBCL.
Conclusions:
Preliminary data demonstrated a tolerable safety profile of ABT-199 in combination with BR across all dosing cohorts. There was no apparent PK interaction between ABT-199 and B; analysis for R is ongoing. Enrollment is ongoing at 400 mg ABT-199 with re-examination of a 28-day cycle schedule with G-CSF prophylaxis in heavily pre-treated patients. Early responses (CR and PR) are seen across all dose cohorts in patients with FL, many of whom have been heavily pretreated. A phase 2 study (NCT02187861; BO29337) of ABT-199 in combination with BR in R/R FL is planned.
Flowers:Genentech: LymphoCare Scientific Advisory Board Other; Celgene: Other; AbbVie, Inc: Research Funding; Acerta: Research Funding; Celgene: Research Funding; Genentech: Research Funding; Gilead Sciences: Research Funding; Infinity Pharmaceuticals: Research Funding; Janssen: Research Funding; Millennium/Takeda: Research Funding; Onyx Pharmaceuticals: Research Funding; Pharmacyclics: Research Funding; Spectrum: Research Funding; OptumRx: Consultancy; Seattle Genetics: Consultancy. Fowler:Roche: Membership on an entity's Board of Directors or advisory committees. Gifford:AbbVie, Inc: Employment. D'Amico:AbbVie, Inc: Employment. Dunbar:AbbVie, Inc: Employment. Zhu:AbbVie, Inc: Employment. Yang:AbbVie, Inc: Employment. Enschede:AbbVie, Inc: Employment. Ricker:AbbVie, Inc: Employment. Chien:AbbVie, Inc: Employment. Humerickhouse:AbbVie, Inc: Employment. Kozloff:Genentech: Consultancy; Roche: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.