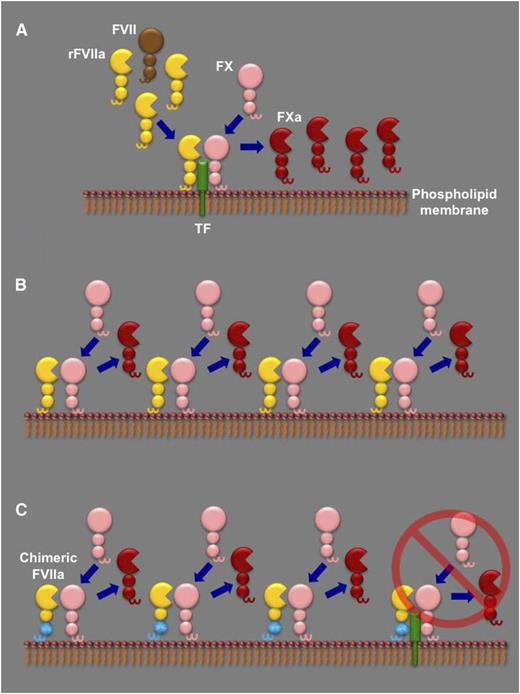
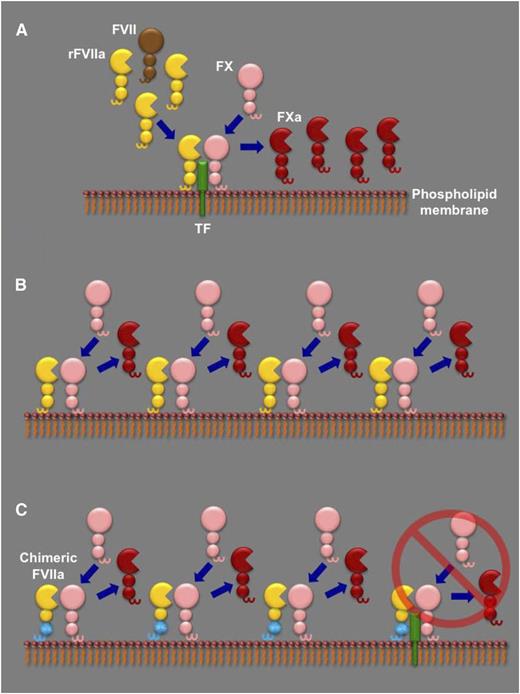
The different proposed models to explain rFVIIa dosing in hemophilia. (A) In the TF-dependent model, high doses of rFVIIa (yellow) compete with endogenous FVII (brown) for binding to their cofactor TF (green) on a phospholipid membrane. The rFVIIa in the TF-rFVIIa complex can then rapidly cleave circulating FX (pink) to its active form (FXa, red), as well as any endogenous FVII complexed with TF (not shown), further enhancing FXa generation. (B) In the TF-independent model, generation of FXa by rFVIIa is essentially by direct FX activation on a phospholipid membrane in the absence of TF. The contribution of the TF-rFVIIa complex is minor (not shown). (C) In the report by Feng et al, the authors generate a chimeric mouse FVIIa molecule that has the Gla and first epidermal growth factor domains from mouse FIX (cyan). As a result, in contrast to wild-type mFVIIa, the chimeric molecule has lost its TF-dependent activity (far right) and can only generate FXa by direct FX activation on a phospholipid membrane. Feng et al report that, when administered in hemophilic mice, both wild-type mFVIIa and the chimeric mFVIIa exhibit similar hemostatic capacity, suggestive of a TF-independent action.
The different proposed models to explain rFVIIa dosing in hemophilia. (A) In the TF-dependent model, high doses of rFVIIa (yellow) compete with endogenous FVII (brown) for binding to their cofactor TF (green) on a phospholipid membrane. The rFVIIa in the TF-rFVIIa complex can then rapidly cleave circulating FX (pink) to its active form (FXa, red), as well as any endogenous FVII complexed with TF (not shown), further enhancing FXa generation. (B) In the TF-independent model, generation of FXa by rFVIIa is essentially by direct FX activation on a phospholipid membrane in the absence of TF. The contribution of the TF-rFVIIa complex is minor (not shown). (C) In the report by Feng et al, the authors generate a chimeric mouse FVIIa molecule that has the Gla and first epidermal growth factor domains from mouse FIX (cyan). As a result, in contrast to wild-type mFVIIa, the chimeric molecule has lost its TF-dependent activity (far right) and can only generate FXa by direct FX activation on a phospholipid membrane. Feng et al report that, when administered in hemophilic mice, both wild-type mFVIIa and the chimeric mFVIIa exhibit similar hemostatic capacity, suggestive of a TF-independent action.
Vascular injury exposes tissue factor (TF) to FVII and traces of FVIIa in blood that bind tightly to the cofactor. The TF-FVIIa complex activates FIX and FX to FIXa and FXa, respectively, and also results in the rapid conversion of the remaining FVII to FVIIa. These reactions constitute key events in the generation of the initiating and the sustained thrombin burst (via FXa and FIXa, respectively). The initial thrombin generation is intact in hemophilia. However, the lack of FVIII or FIX and the rapid inhibition of the TF-FVIIa complex by TF pathway inhibitor prevents sustained thrombin generation. Moreover, hemophiliacs develop inhibitory antibodies to infused FVIII or FIX, preventing the correction of hemostasis by protein replacement. Such patients can be managed by so-called bypass agents, termed for their ability to effect hemostasis even in the presence of inhibitory antibodies. rFVIIa is an effective bypass agent indicated for hemophilia patients with inhibitors. In these patients, the therapeutic rFVIIa dose of 90 µg/kg every 2 to 3 hours (until hemostasis is achieved) raises the plasmatic FVIIa levels by 250-fold (∼25 nM) of normal. Surprisingly, similar rFVIIa dosing is also used in nonhemophilic patients who require hemostatic correction, suggesting that FVIII or FIX deficiency alone cannot account for the need for high doses of rFVIIa required in hemophilia.
Why are high doses of rFVIIa necessary in hemophilia? To address this, one has to look at the biochemical intricacies of FVIIa: it is a poor enzyme that requires both TF and phospholipid membrane binding to exert its function in normal hemostasis. These requirements have formed the basis of two models to explain the dosing of rFVIIa in hemophilia, as shown in panel A-B. The first suggests that a high dose of rFVIIa is necessary to compete with endogenous FVII for binding to TF; thus, rFVIIa effects hemostasis in a TF-dependent manner.2 The second pivots on the poor capacity of FVIIa to activate FX in the absence of TF; high rFVIIa doses can overcome this defect and thus result in hemostasis in a TF-independent manner.3,4 Recent mathematical modeling and in vitro data have demonstrated the nonexclusive nature of each model but ultimately suggested a predominantly TF-dependent mechanism of action.5 However, there are 2 clinical observations that are still unexplained: first, increasing the rFVIIa dose should not increase efficacy if TF is a limiting factor, yet this is observed in some patients receiving rFVIIa doses >200 µg/kg.6 Second, an rFVIIa analog with enhanced TF-independent activity (but normal TF-dependent activity) compared with wild-type rFVIIa has exhibited increased clinical efficacy.7
This impasse in interpretation is further complicated by the fact that the γ-carboxyglutamic acid (Gla) domain of FVII/FVIIa is involved in both TF and phospholipid membrane binding. Feng et al hypothesize that if one could generate an rFVIIa molecule lacking only functional TF binding, then comparing its bypassing capacity to wild-type rFVIIa in hemophilia would reveal the contribution of TF to function. They test their hypothesis in hemophilic mice using a 1-2–punch approach. First, they generated a chimeric mouse FVIIa molecule that contained the Gla and first epidermal growth factor domains from mouse FIX (mFIXgla-egf1-mFVIIa). This molecule lacks TF-dependent activity but retains the ability to activate FX to the same extent as wild-type mFVIIa. Essentially, the overall hemostatic function of mFIXgla-egf1-mFVIIa should be devoid of a TF contribution (panel C). The second part of their approach used a mouse injury model (saphenous vein bleeding) where hemostasis was sensitive to levels of endogenous TF. This allowed them to use this injury model in hemophilic mice and compare the hemostatic capacity of wild-type mFVIIa to that of mFIXgla-egf1-mFVIIa. If the hemostatic effect of protein infusion had a TF component, then mFIXgla-egf1-mFVIIa should be less effective than wild-type mFVIIa. They report that both molecules were equally effective and could both normalize the hemophilic phenotype in that specific assay.
The data from Feng et al provide strong in vivo support for the capacity of rFVIIa to restore hemostasis in hemophilia independently of TF. However, as the authors note, the pharmacokinetic (PK) properties of mFIXgla-egf1-mFVIIa were not investigated. Because the Gla domain of FIX can also bind subendothelial collagen IV, it is possible that the effects of mFIXgla-egf1-mFVIIa could also be an outcome of a different PK profile to mFVIIa. Further studies are needed to answer this question. In addition to TF, other receptors have also been shown to interact with human rFVIIa, such as platelet GPIbα8 and the endothelial protein C receptor,9 with unclear hemostatic function in vivo. They may play a role in the localization of the procoagulant reactions in which infused rFVIIa participates. For example, the activated endothelium can support functional prothrombinase complex assembly.10 It is tantalizing to think that, in addition to platelets, some of the rFVIIa procoagulant effects in hemophilia may be mediated by reactions on the activated endothelial surface. Further clarification of the mechanism of action of rFVIIa as a bypassing agent will require these points to be addressed in future studies.
The mechanistic insights provided by Feng et al may form the rational basis for the design of novel rFVIIa variants with more favorable properties as bypass agents. However, one must consider that domain swapping in rFVIIa may break the immunologic tolerance to the existing recombinant as well as endogenous protein. Structural changes by mutagenesis that enhance the TF-independent activity of rFVIIa have already encountered this problem in human patients and halted further product development. Subtle sequence changes may alleviate the immunologic problems but will certainly be harder to conceive and properly evaluate. Moreover, rFVIIa is used extensively off-label as an emergency agent to control bleeding in adults and pediatric populations, with some reported thrombotic complications. Therefore, the properties of any novel rFVIIa molecules with respect to the balance of safety and efficacy will need to be carefully considered.
Conflict-of-interest disclosure: P.M. received research funding through competitive grants from the Bayer Hemophilia Awards Program, additional research funding from Novo Nordisk A/S, and a salary (spouse) from Shire Inc. and Bristol-Myers Squibb.