In this issue of Blood, Savchenko et al1 demonstrate that removal of neutrophil extracellular traps (NETs) and inhibition of neutrophil recruitment by DNase I with or without ADAMTS13 or inactivation of peptidyl arginine deiminase 4 (iPAD4) protects from cardiac damage after myocardial infarction in mice.2
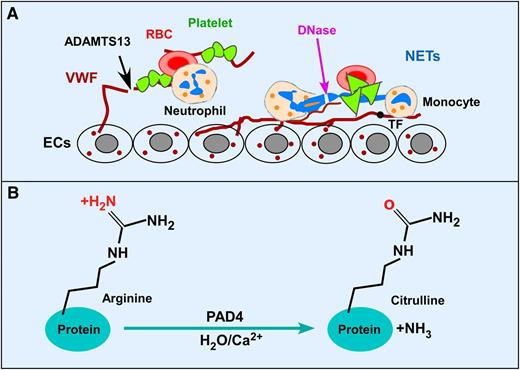
Potential therapeutic targets and citrullination by PAD4 after MI. (A) Activated endothelium releases VWF that binds circulating platelets and leukocytes and promotes thrombosis and inflammation after acute MI. ADAMTS13 cleaves VWF and inhibits platelet aggregation and early leukocyte recruitment and NET formation. NETs released from activated neutrophils or dying cardiomyocytes are prothrombotic by trapping platelets and red blood cells, and binding tissue factor. DNase I, which degrades NETs, is protective against cardiac injury. (B) PAD4 catalyzes imine to ketone reactions in a Ca2+-dependent manner on specific peptidyl arginine side groups of nuclear proteins, such as histones H3 and H4, a process known as citrullination or deimination. This process is required for the NET formation. Inactivation of PAD4 enzymatic activity genetically or pharmacologically inhibits thrombosis and inflammatory response and may be protective against cardiac damage after acute MI. ECs, endothelial cells; RBC, red blood cell; TF, tissue factor.
Potential therapeutic targets and citrullination by PAD4 after MI. (A) Activated endothelium releases VWF that binds circulating platelets and leukocytes and promotes thrombosis and inflammation after acute MI. ADAMTS13 cleaves VWF and inhibits platelet aggregation and early leukocyte recruitment and NET formation. NETs released from activated neutrophils or dying cardiomyocytes are prothrombotic by trapping platelets and red blood cells, and binding tissue factor. DNase I, which degrades NETs, is protective against cardiac injury. (B) PAD4 catalyzes imine to ketone reactions in a Ca2+-dependent manner on specific peptidyl arginine side groups of nuclear proteins, such as histones H3 and H4, a process known as citrullination or deimination. This process is required for the NET formation. Inactivation of PAD4 enzymatic activity genetically or pharmacologically inhibits thrombosis and inflammatory response and may be protective against cardiac damage after acute MI. ECs, endothelial cells; RBC, red blood cell; TF, tissue factor.
Acute myocardial infarction (MI), resulting from intraluminal coronary thrombosis after rupture of an atherosclerotic plaque, is the leading cause of death in the United States and worldwide. Current therapy aims at early restoration of coronary blood flow by using primary percutaneous coronary intervention and/or thrombolysis.2 However, these approaches do not adequately address reperfusion injury occurring shortly after an ischemic event. Recruitment of neutrophils and monocytes into the infarct site results in the formation of NETs that contain DNA, citrullinated histones H3 and H4, and granule cytotoxic enzymes.3 NETs are shown to play a role in innate immunity by killing microbes and preventing their spread. NETs also serve as scaffolds that trap flowing platelets, leukocytes, and red blood cells from circulation (see figure, panel A). Furthermore, NETs can trigger the release of von Willebrand factor (VWF) from Weibel-Palade bodies in endothelial cells and inactivate tissue factor pathway inhibitor (see figure, panel A), thereby enhancing platelet aggregation, thrombin generation, and fibrin formation. Therefore, NETs are proinflammatory and prothrombotic. Targeting NETs and VWF simultaneously may offer a greater therapeutic benefit in patients with arterial and venous thrombosis.
Using a murine acute MI/reperfusion injury model, Savchenko et al elegantly demonstrate that administration of DNase I with or without recombinant human ADAMTS13 (rhADAMTS13) significantly reduces infarct size, neutrophil recruitment/infiltration, and plasma levels of nucleosomes, thereby improving cardiac function.1 Interestingly, their results also show that rhADAMTS13 is slightly more efficacious than DNase I, but a combination of DNase I and rhADAMTS13 appears to be significantly more efficacious in preserving myocardial function than either one alone.1 These results indicate that targeting early VWF-mediated leukocyte recruitment by ADAMTS13 and later formation of NETs by DNase I may offer significant protection against myocardial damage in this model.
Previous study has shown that the early recruitment of neutrophils and monocytes to the site of ischemic injury is highly dependent on endothelial VWF. For instance, mice lacking VWF (vwf−/−) exhibit significantly reduced infarct size and neutrophil infiltration at the site of injury compared with those with normal levels of VWF.4 Conversely, mice lacking ADAMTS13 (Adamts13−/−), a plasma metalloprotease that cleaves VWF, exhibit the opposite effects after cardiac ischemia/reperfusion injury.4,5 Moreover, treatment of mice with a neutralizing antibody against VWF or infusion of rhADAMTS13 significantly reduces the infarct size and improves cardiac function.1,4,5 These findings support a pivotal role for the VWF/ADAMTS13 axis in the pathogenesis and therapeutics of acute MI.
NET formation requires peptidyl arginine deiminase 4 (PAD4), which is highly expressed in the nucleus of neutrophils, monocytes, and macrophages.6,7 Upon stimulation by chemokines or bacteria and other host factors, PAD4 catalyzes the conversion of an arginine residue to citrulline on the tail of histone H3 and H4,8 a process referred to as citrullination or deimination (see figure, panel B). Neutrophils with genetic inactivation of PAD4 (iPAD4) cannot form NETs after stimulation and are deficient in bacterial killing.9 Here, Savchenko and colleagues convincingly demonstrate that mice with iPAD4 are equally protected from cardiac damage after acute MI/reperfusion injury as shown by a dramatic reduction in infarct size, neutrophil infiltration, citrullinated H3, and release of nucleosomes in plasma.1 The presence of citrullinated H3 in situ and increased levels of plasma nucleosomes is suggestive of NET formation after an injury. In a previous report, mice with iPAD4 exhibit similar protective effects against experimental deep vein thrombosis.10
Together, the findings from Savchenko and colleagues demonstrate for the first time that VWF-mediated leukocyte recruitment by PAD4 exacerbates cardiac damage after MI/reperfusion injury. Combined therapy with ADAMTS13 and DNase I or a PAD4 inhibitor to eliminate the initial platelet and neutrophil/monocyte recruitment and the subsequent NET formation may represent a novel therapeutic strategy for acute MI in humans.
Conflict-of-interest disclosure: The author declares no competing financial interests.