Although autologous stem cell transplantation (ASCT) is effective for patients with multiple myeloma (MM), disease relapse remains problematic. Despite significant advances in therapeutics for MM, it remains mostly incurable. Following ASCT, a majority of MM patients are in a minimal residual disease state, at which time delivery of immunotherapy may be most effective. Regulatory T cells (Tregs) are a naturally-suppressive CD4+ T cell population (CD4+CD25+FoxP3+ T cells) that expand in MM patients, and strongly inhibit anti-tumor immune responses. In pre-clinical models Treg depletion enhanced the function of tumor antigen-specific T cells. Effective strategies to deplete Tregs in humans are being investigated.
We initiated pilot study to test 2 methods of Treg depletion in MM patients undergoing ASCT. In the first approach, an anti-CD25 monoclonal antibody (basiliximab) was administered day +1 following ASCT (in vivo Treg depletion). The second method involved depleting CD4+CD25+ Tregs from autologous stem cell (ASC) grafts with clinical-grade anti-CD25 microbeads and the CliniMACS device (Miltenyi) (ex vivo Treg depletion). A control arm consisted of patients in which no Treg-depleting maneuver was performed. To date, 10 patients with symptomatic MM have been enrolled and randomly assigned to 1 of 3 study arms (arm 1 – standard ASCT; arm 2 – in vivo Treg depletion; arm 3 – ex vivo Treg depletion). Primary endpoints included: a) efficacy of ex vivo Treg depletion from ASC grafts, b) kinetics of Treg depletion and recovery in each study arm, and c) assessment of toxicity associated with Treg depletion. Secondary endpoints included time to engraftment following ASCT and disease response. The overall goal of the study was to identify a superior strategy with regard to depth and durability of Treg depletion.
Ten patients (median age 59; range 46-68) have been enrolled. 4 were enrolled onto arm 1, 3 onto arm 2 and 3 onto arm 3. One patient enrolled on arm 2 was removed from study due to ASC mobilization failure. ASC were collected following neupogen and plexifor, and conditioning for ASCT consisted of melphalan (200mg/m2). Typical serious adverse events associated with ASCT were observed in all study arms, with neutropenic fever being the most common (n=2 arm 1, n=1 arm 2 and n=2 arm 3). Autoimmune complications, such as autologous graft-versus-host disease, were not observed. All patients engrafted post-ASCT with normal kinetics. Too few patients have been treated to make conclusions regarding clinical efficacy.
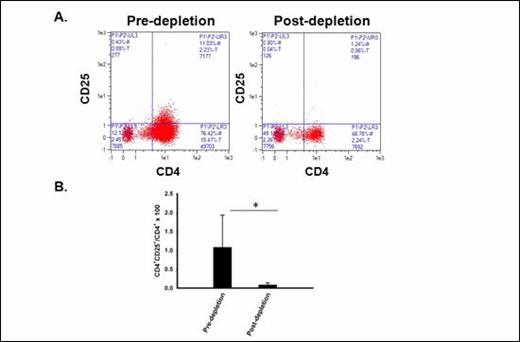
Treg are effectively depleted ex vivo with CD25 microbeads and the Clinimacs device. A FACS plots showing frequency of CD4 +CD25 + Tregs before (left) and after (right) ex vivo Treg depletion procedure (arm 3). B Mean CD4+CD25+ Treg frequencies before and after ex vivo Treg depletion (*p= 0.05).
Treg are effectively depleted ex vivo with CD25 microbeads and the Clinimacs device. A FACS plots showing frequency of CD4 +CD25 + Tregs before (left) and after (right) ex vivo Treg depletion procedure (arm 3). B Mean CD4+CD25+ Treg frequencies before and after ex vivo Treg depletion (*p= 0.05).
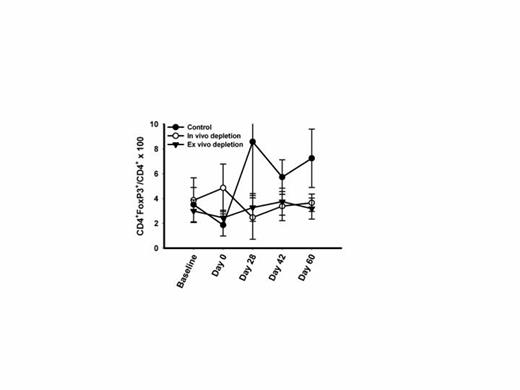
Kinetics of Treg depletion following ASCT. Mean (+/- SD) frequency of CD4+FoxP3+ Tregs before and after ASCT in each study arm are displayed.
Kinetics of Treg depletion following ASCT. Mean (+/- SD) frequency of CD4+FoxP3+ Tregs before and after ASCT in each study arm are displayed.
In conclusion, both the in vivo or ex vivo method of Treg depletion appear to be effective based on small patient numbers. Treg depletion in MM patients undergoing ASCT is safe and does not delay engraftment. Additional patients will need to be treated to make conclusions regarding clinical efficacy. Future studies of Treg depletion in combination with other immunotherapeutic strategies are being considered.
No relevant conflicts of interest to declare.