Abstract
Secondary mutations in BCR-ABL are the most common cause of resistance to TKIs in patients (pts) with chronic myeloid leukemia (CML). Ponatinib is a potent pan-BCR-ABL TKI that has been shown to suppress the emergence of any single mutation in vitro, including T315I, at clinically achievable concentrations (40 nM), though higher concentrations were required to suppress emergence of certain compound mutations (2 mutations in the same BCR-ABL allele). Ponatinib has demonstrated significant clinical activity in pts in the phase 2 PACE trial, 60% of whom received 3 or more prior TKIs. Responses were observed for each of the 15 mutations present in >1 chronic phase CML pt at baseline, and no single mutation conferring resistance to ponatinib has emerged to date, though in some cases development of compound mutations has been observed. To gain a more precise understanding of the effects of specific mutations on the clinical efficacy of ponatinib, IC50s for ponatinib, and all other approved TKIs, against 31 single or compound BCR-ABL mutants were determined. To explore the relationship between in vitro potency and clinical efficacy, IC50s were related to “effective” TKI levels achieved in patients.
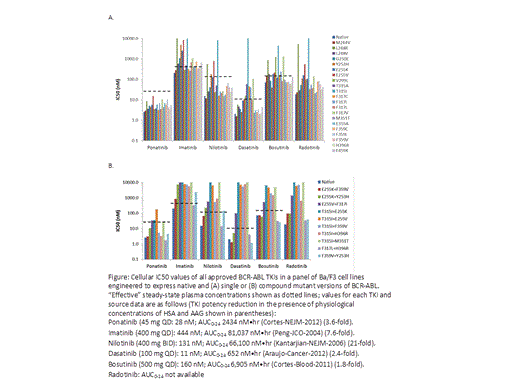
TKI potency was assessed in engineered Ba/F3 cells by measuring cell viability at 72 hours. The effective plasma concentration for each TKI was calculated from published average steady-state concentration values for the recommended dose, and adjusted for the functional effects of protein binding. These effects were assessed by determining the degree to which TKI potency was reduced by the presence of physiological concentrations of human serum albumin (HSA) and alpha 1-acid glycoprotein (AAG).
The activity of ponatinib, and 5 other TKIs, against 21 single BCR-ABL mutants is shown in Figure A. Ponatinib potently inhibited viability of native BCR-ABL and all mutants, including T315I (IC50s (nM): 3-16). The IC50s for the other TKIs, excluding T315I (>4000 for all) ranged from: 201-10,000 (imatinib), 12-784 (nilotinib), 2-104 (dasatinib), 40-1,280 (bosutinib) and 18-5,216 (radotinib). IC50 values were compared to the effective plasma concentration for each TKI (Figure A). Mutants that have previously been associated with clinical resistance to a particular TKI tended to have IC50s that approached or substantially exceeded the effective concentration for that TKI, including most mutants for imatinib, E255K/V, Y253H, L248R, T315I for nilotinib, and V299L, F317C/I/V, T315A/I for dasatinib. The most problematic mutants for bosutinib predicted by this analysis were F317V, L248R, V299L, and T315I. Notably, all mutant IC50s fell below the effective concentration for ponatinib. The activity of all TKIs against 10 clinically-observed BCR-ABL compound mutants was also assessed. Four compound mutants had IC50s near or above the effective concentration for ponatinib (T315I+M351T, E255V+F317I, T315I+E255K, T315I+E255V) (Figure B). All 4, plus others, are also predicted to be problematic for the other TKIs.
Relating in vitro TKI potency to “effective” steady-state plasma concentrations in patients identified mutations known to confer clinical resistance to imatinib, nilotinib and dasatinib. This method of analysis suggests that ponatinib may be able to inhibit all single BCR-ABL mutants, but not all compound mutants, a prediction that is thus far consistent with results observed in patients. Compound mutations that are predicted to confer resistance to ponatinib are also predicted to confer resistance to all other approved TKIs. Early introduction of ponatinib may prevent the emergence of single mutations, and thus the sequential development of compound mutations.
Gozgit:ARIAD: Employment, Equity Ownership. Schrock:ARIAD: Employment, Equity Ownership. Chen:ARIAD: Employment, Equity Ownership. Clackson:ARIAD: employees of and own stock/stock options in ARIAD Pharmaceuticals, Inc Other, Employment. Rivera:ARIAD: Employment.
Author notes
Asterisk with author names denotes non-ASH members.