Abstract
Dabigatran etexilate (DE) is an oral direct thrombin inhibitor approved for prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation (NVAF). In the RE-LY trial, myocardial infarction (MI) rates were increased with DE 110mg bid and 150mg bid when compared to warfarin. The risk of MI associated with the use of DE was assessed in a previous meta-analysis of 7 non-inferiority randomized controlled trials (RCTs) showing a significant 33% increase in MI.
We performed an updated meta-analysis of RCTs comparing DE with active comparators or placebo to assess the effect of this agent on MI risk as a primary objective. The outcome of major bleeding (MB) and all-cause mortality was also assessed to provide global safety and efficacy measure. Stratifications by comparators (enoxaparin, warfarin or placebo) and by studies using the 150mg bid and the 110mg bid dose regimen were performed.
We conducted searches of the published literature and a clinical-trials registry maintained by the drug manufacturer till 22th of March, 2013. Criteria for inclusion in our meta-analysis included all RCTs and the availability of outcome data for MI, MB and all-cause mortality. Among the 423 unique references identified, 13 RCTs fulfilled the inclusion criteria. All methodologies were performed according to the PRISMA Statement.
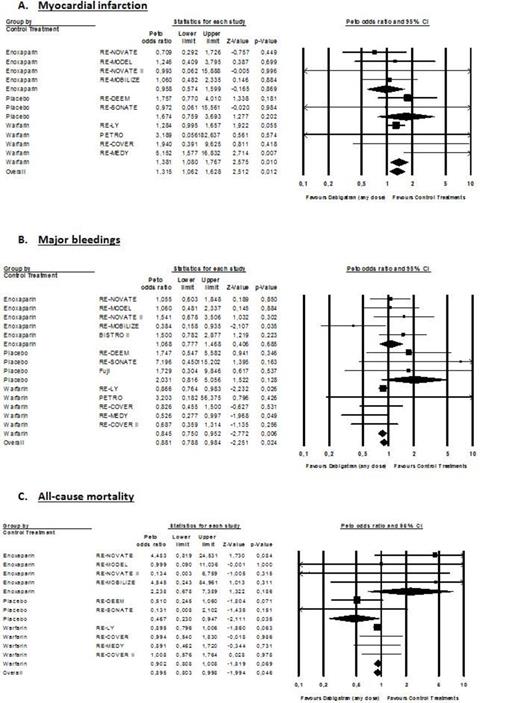
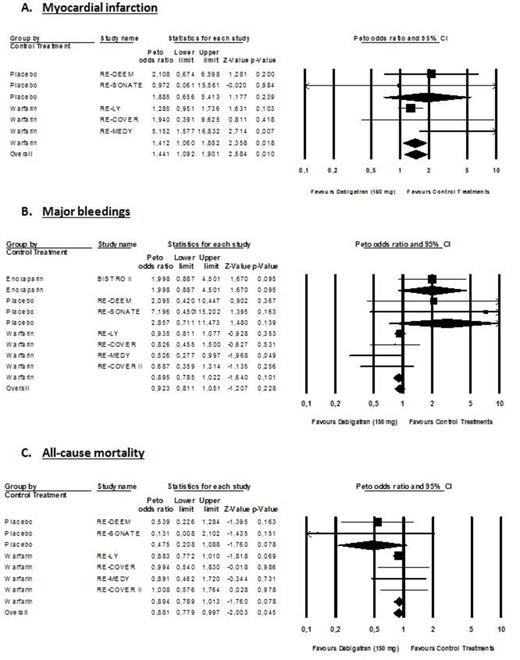
Myocardial infarction occurs in 287 of 23,839 patients (1.20%) treated with DE and in 106 of 13,536 patients treated with controls (0.78%). Major bleeding occur in 948 of 27,063 patients (3.50%) treated with DE and in 551/15,341 patients (3.59%) treated with controls. Death occurs in 989 of 24,162 patients (4.09%) treated with DE and in 570 of 14,498 patients (3.93%) treated with controls. Overall OR for MI, MB and all-cause mortality were 1.32 (95% CI; 1.07-1.63; P=0.010), 0.85 (95% CI: 0.79-0.98; P=0.010) and 0.90 (95% CI; 0.81-1.00; P=0.046) (Figure 1). When compared to warfarin, OR for MI, MB and all-cause mortality were 1.38 (95% CI: 1.08-1.77; P=0.010), 0.85 (95% CI: 0.75-0.95; P=0.006) and 0.90 (95% CI: 0.81-1.01; P=0.069), respectively (Figure 1). In RCTs using the 150mg bid dose regimen, OR for MI, MB and all-cause mortality were 1.44 (95% CI: 1.09-1.90; P=0.010), 0.92 (95% CI: 0.81 to 1.05; P=0.228) and 0.88 (95% CI: 0.78-1.00; P=0.045), respectively (Figure 2). Results of the 110mg bid dose were mainly driven by the RE-LY trial.
DE significantly reduced MB and all-cause mortality compared to controls. However, while the reduction of MB is statistically significant versus warfarin, the reduction in all-cause mortality is not (Figure 1B & 1C). The increased risk of MI with the 150mg bid dose is significant but the reduction in MB and mortality is non-statistically significant (Figure 2). Taken together, these remarks suggest that in frail patients presenting comorbidities, the choice of the 150mg bid dose should be carefully discussed and the 110mg bid dose might be considered. Based on our results, one cannot conclude that the 110mg bid dose is associated with a higher risk of MI.
However, in terms of absolute risk, such an increased risk of MI should be tempered when compared to the outcomes of stroke or systemic embolism, MB and all-cause mortality. The results from the RE-LY trial showed that the benefits of DE over warfarin outweigh this increase risk of MI. The risk difference was greatly in favor of DE regarding the composite of stroke/systemic embolism, MI, MB and all-cause mortality.
This meta-analysis of RCTs provides robust evidence that DE is associated with an overall significant 32% increase in the risk of MI. The risk was principally identified when warfarin is used as comparator (38% increase). In RCTs using the 150mg bid DE dose, a significant 44% overall increased risk of MI was identified. No definitive conclusion about the absence of the risk of MI with the 110mg bid DE dose can be drawn at this time. However, this increase risk has to be tempered with the overall benefit of DE especially in the patients with NVAF. In conclusion, we suggest that health care professionals and regulators should consider additional risk minimization strategy to prevent the risk of MI in vulnerable population.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract