Abstract
Proteasome inhibitors have become an important part of myeloma therapy along with the IMiDS, but both approved drugs require parenteral administration. An orally bioavailable proteasome inhibitor will significantly enhance the ability to incorporate this class of drugs in majority of patients, and increase the ability to develop oral combinations. MLN9708 is an orally bioavailable proteasome inhibitor, that has been shown to have activity in relapsed MM, and in combination with lenalidomide has been effective for newly diagnosed MM. However, the vast majority of patients in the prior trial had relapsed after bortezomib or was refractory to bortezomib. We designed this trial to evaluate the single agent activity of MLN9708 in patients with limited exposure to bortezomib who were not refractory to that agent.
We designed a phase 2 trial of MLN9708, used a single agent, in patients with relapsed myeloma who had received none or less than 6 cycles of a bortezomib based regimen as part of their prior treatment. MLN9708 was given at a flat dose of 5.5 mg once weekly for three out of four weeks. Dexamethasone (20 mg on the day of and the day after MLN) was added for lack of a minor response by end of Cycle 2 or lack of a partial response by end of Cycle 4 or if there was disease progression at any time. Responses were assessed by the IMWG criteria and toxicity was graded using the CTCAE v4.0. The primary endpoint of this study is the proportion of confirmed responses with single agent MLN9708.
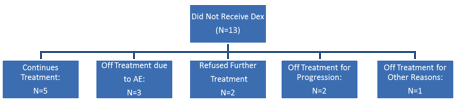
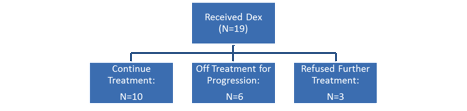
Thirty-three patients were enrolled, and one patient was considered ineligible and excluded from all analysis. The median age was 69 years and 53% were male. The median duration from diagnosis was 57 mos (range 14 mos to 12.3 years) and patients had a median of 2 prior therapies (range 1-7). Prior therapies included IMiDs (88%), bortezomib (28%) and stem cell transplant (59%). At the time of data cutoff, 75% of patients had not progressed and all but 2 (94%) were alive, with a median follow up of 7 months. Nearly half (47%) are still on therapy; reasons for drug discontinuation were disease progression (8), refusal (5) and adverse event (3). Overall, 184 cycles of treatment were delivered, with a dose reduction required for 15 (8%) cycles. A grade 3 or 4 adverse event considered at least possibly related to drug was seen in 18 (56%) and 6 (19%) patients, respectively; there were no deaths on study. The most common adverse events observed included thrombocytopenia, fatigue, nausea, and diarrhea. Peripheral neuropathy possibly related to the drug was seen in 6 patients (grade 1) and 2 patients (grade 2), respectively. Dexamethasone was initiated in 19 (59%) patients, 16 for not reaching the desired response and 3 for progression. Response (>=PR) to single agent was seen in 5 patients within 4 cycles of therapy including 3 patients with PR and 2 patients with a stringent CR. Six additional patients who had either an MR (2) or SD (4) achieved a PR after addition of dexamethasone, translating to an overall response rate of 34% (11/32). The median event free survival was 12.4 months and 6 month overall survival was 96%. The patient disposition by addition of dexamethasone is shown in the figure.
MLN9708 as a single agent results in a 16% PR or better after 4 cycles of therapy. RR improved to 34% with the addition of dexamethasone in patients not responding to single agent MLN9708. This includes 2 patients with a sCR on single agent MLN9708. We anticipate the response rate and response depth to improve with nearly half of the patients still remaining on therapy.
Kumar:Merck: Consultancy, Honoraria; Celgene: Consultancy, Research Funding; Millennium: The Takeda Oncology Company: Research Funding; Novartis: Research Funding; Genzyme: Research Funding. Reeder:Celgene: Research Funding; Novartis: Research Funding; Millenium: Research Funding. Lacy:Celgene Corporation: Research Funding. Gertz:Celgene: Honoraria. Dispenzieri:Celgene, Millenium, Jansenn, Pfizer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.