Abstract
Sevuparin is a new chemically modified heparin with low anticoagulant activity currently being studied as an adjuvant therapy for severe malaria. Heparins generally have multiple biological properties, including antithrombin III-dependent inhibition of thrombin as well as blockade of P-selectin-mediated adhesion. Selectins have been shown to contribute to both sickle red cell (SS RBC) and neutrophil (PMN) adhesion in vitro and in mice with sickle cell disease (SCD). Sickle mice lacking both P- and E-selectins are relatively resistant to tumor necrosis factor-α (TNFα)-induced vaso-occlusion. We therefore theorized that sevuparin would show activity in inhibiting the selectin-dependent adhesion of red cells and leukocytes seen in the context of SCD, with the potential to decrease vaso-occlusion at a low level of anti-coagulation.
We studied both RBC and PMN adhesion to human umbilical vein endothelial cells (ECs) in an in vitro flow chamber. For studies of SS RBC adhesion, we exposed confluent cultures of ECs on gelatin-coated glass slides to interleukin (IL)-13 (50 ng/ml) for 48 h, followed by histamine (100 μM) for 10 min at 37°C immediately prior to performance of adhesion assays. Upregulation of P-selectin expression was confirmed by FACS of mechanically dislodged ECs. RBC adhesion was quantified during continuous flow. For assays of neutrophil adhesion, ECs were treated with human TNFα (10 μg/ml) overnight, followed by incubation for 10 min at 37°C with 100 µM histamine. All slides bearing ECs were washed after incubation with histamine and immediately mounted in the flow chamber for adhesion assays. Inhibition by sevuparin was tested by pre-incubating ECs with different concentrations of sevuparin for 20 min at 37°C prior to adhesion assays. PMNs from SCD patients were tested for adhesion to treated ECs with and without preceding exposure of ECs to sevuparin. In addition, PMNs from normal donors were first exposed to SS RBCs, as previously described, and then tested for adhesion to ECs with and without pre-exposure to sevuparin. PMN adhesion was quantitated after 10 min of no flow, followed by wash out at various shear stresses. The ability of sevuparin to prevent vaso-occlusion in vivo was studied by infusing red fluorescent SS RBCs into nude mice to effect in vivo adhesion and vaso-occlusion visible in implanted window chambers (Zennadi et al. 2007); 500 ng of murine TNFα in 100 μl was injected intraperitoneally (IP) 4 h before infusion of SS RBCs to increase endothelial expression of selectins. Sevuparin or saline control solution was injected SQ at various dose levels prior to infusion of SS RBCs into nude mice.
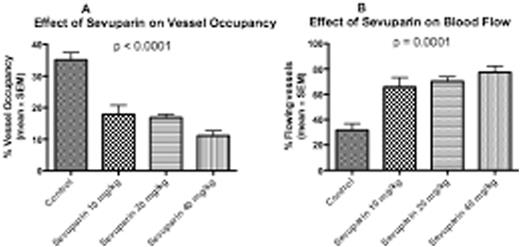
SS RBC adhesion to ECs stimulated with IL13 and histamine was greater than adhesion to similarly stimulated ECs pretreated with sevuparin at 100, 200, 400 and 600 µg/ml prior to exposure to flowing SS RBCs (p = 0.047, 0.031, 0.094, 0.065, respectively, using a paired t test, in which each patient sample was only compared to itself), with a robust dose-response (p < 0.001) (Fig 1A). In a similar analysis, 7.5 µg/ml of function blocking monoclonal antibody to CD62P (9E10) also significantly reduced RBC adhesion (p = 0.038). Sevuparin significantly inhibited adhesion of SCD PMNs to ECs treated with TNFα and histamine, and this inhibition exhibited a modest dose-response relationship (Fig 1B). When normal PMNs stimulated by SS RBCs were studied, the differences between adhesion in the absence of sevuparin and in the presence of either 600 or 800 µg/mL sevuparin were also highly significant at both 1 and 2 dyne/cm2 (p = 0.019 and p = 0.011 at 1 dyne/cm2 and p = 0.013 and p = 0.008 at 2 dynes/cm2, respectively) (Fig 1C). In vivo, injection of sevuparin prior to infusion of SS RBCs significantly decreased SS RBC adhesion to vessel walls, as measured by the percent of vessel lengths occupied by adherent cells (Fig 2A). Sevuparin treatment also significantly increased the percent of venules that maintained normal blood flow (Fig 2B).
Sevuparin is an effective inhibitor of SS RBC adhesion and both SCD and normal PMN adhesion to endothelial cells in vitro. In vivo, sevuparin effectively decreased vaso-occlusion and improved blood flow after TNFα treatment. Therefore, we consider sevuparin a promising anti-adhesion agent with potential to reduce vaso-occlusion in SCD, via reducing RBC adhesion and leukocyte adhesion, possibly through its effect on selectins.
Batchvarova:Dilaforette, AB: Research Funding. Shan:Dilaforette, AB: Research Funding. Zennadi:Dilaforette, AB: Research Funding. Lindgren:Dilaforette, AB: Employment. Leitgeb:Dilaforette, AB: Employment. Sulila Tamsen:Dilaforette, AB: Employment. Telen:GlycoMimetics, Inc.: Research Funding; Dilaforette, NA: Research Funding; Pfizer, Inc.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.