Abstract
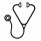
Maximum grades of the commonly used modified Glucksberg staging system for acute graft versus host disease (GVHD) correlate with non-relapse mortality (NRM), but cannot guide treatment at diagnosis. All patients with GVHD grade II-IV are standardly treated with high dose steroids, and no further treatment decisions are based on GVHD severity at onset. In contrast, treatment for GVHD grade I varies so that some patients receive systemic steroid therapy while others are observed. We wished to devise a grading system that predicted eventual mortality and that could therefore guide treatment at diagnosis. We have previously shown that plasma concentrations of GVHD biomarkers (TNFR1, IL2Rα, elafin, REG3α, ST2) have prognostic significance (Paczesny, Blood, 2009, Paczesny, Sci Trans Med, 2010, Ferrara Blood, 2011, Vander Lugt, NEJM, 2013). We identified 360 University of Michigan allogeneic hematopoietic cell recipients with acute GVHD (median age 48y, range 0-70y) who had plasma samples taken at the time of diagnosis [median day 28, range: 5-174] and stored in our repository: grade I (n =130, 36%), grade II (n = 151, 42%), or grade III/IV (n = 79, 22%). Biopsy confirmation was available for >80% of cases. Progression from Glucksberg I to Glucksberg ≥ II occurred in 84 (64%) patients at a median of 6 days from onset, whether systemic therapy for grade I was initiated (N=80; 70%) or not (N=50; 56%, p=0.12). We chose 6 month NRM as the key endpoint as it encompasses treatment failures due to nonresponse, loss of treatment response, and complications of treatment; 93% of 6 m NRM was due to GVHD. The 6 m NRM for patients presenting with Glucksberg I (15%) and Glucksberg II (24%) was not significantly different [as also shown by others: MacMillan, Blood, 2010]; their combined NRM was 20% (n=281, Panel A). Patients who presented with Glucksberg III/IV had significantly worse 6 m NRM than Glucksberg I/II (56%, N=79, p<0.001, Panel C). We measured the plasma concentrations of the 5 prognostic biomarkers above and used logistic regression to develop Ann Arbor GVHD grades such that 6 m NRM for Ann Arbor I would be ∼10% (as in patients with Glucksberg I whose GVHD never progressed) and >50% for Ann Arbor III (similar to the 6 m NRM for Glucksberg III/IV). We treated relapse after development of GVHD as a competing risk and developed separate regression models according to the clinical severity at presentation, i.e. Glucksberg I/II and III/IV. Panel B shows the 6 m NRM of patients who presented with Glucksberg I/II according to Ann Arbor I (thin), II (medium), and III (thick). Patients with Ann Arbor III (n=29/281, 10%) experienced far worse 6 m NRM (59%) than either Ann Arbor I (11%) or Ann Arbor II (17%) [p<0.001 for each]. Likewise, Panel D shows that biomarkers reclassify significant numbers of patients with Glucksberg III/IV at onset to Ann Arbor I and II (n=21/79, 27%). These reclassified patients experienced much lower NRM (0% and 21%, respectively) than patients with Ann Arbor III (71%, p<0.001). Results are summarized in Panels E (combined Glucksberg NRM) and F (combined Ann Arbor NRM). NRM for Ann Arbor grade III remained significantly worse compared to Ann Arbor grade I and II (p<0.001 for both comparisons). Of note, the difference between Ann Arbor grade I and II (10% vs 18%, p=0.08) approaches statistical significance. Comparison of the models by the Akaike Information Criterion indicated that Ann Arbor grades predict NRM better than Glucksberg grades. Patients with Ann Arbor grade III GVHD experienced worse NRM (67%) than those with Glucksberg III/IV (56%), while patients with Ann Arbor grade I (10%) have better NRM than those with Glucksberg I/II (20%). As expected, patients with Ann Arbor grade I were most likely to respond to treatment by day 28 and those with Ann Arbor III GVHD were least likely (p=0.01 and p=0.03, respectively). In conclusion, we have developed a new acute GVHD grading system using biomarkers at onset of disease that reclassifies significant numbers of patients and produces more accurate risk groups than Glucksberg grades. If validated in patients from other centers, this system may be able to guide therapy: patients with Ann Arbor Grade III who experience high rates of treatment failure with standard approaches would benefit from experimental therapies as primary treatment. Likewise, patients with Ann Arbor grade I might require low dose/no systemic steroids.
Disclosures:
Levine:University of Michigan: Patent for GVHD biomarkers, Patent for GVHD biomarkers Patents & Royalties. Braun:University of Michigan: Patent for GVHD Biomarkers, Patent for GVHD Biomarkers Patents & Royalties. Ferrara:University of Michigan: Patent for GVHD Biomarkers, Patent for GVHD Biomarkers Patents & Royalties.
Author notes
*
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract
© 2013 by The American Society of Hematology
2013