Abstract
The assessment of residual tumor cells persisting after therapy, or minimal residual disease (MRD), is a central component of accurate disease prognosis in acute lymphoblastic leukemia (ALL) (Pui et al, JCO 2011). MRD assessment has recently been shown to be useful for monitoring disease before and after stem cell transplantation and during salvage therapy for early detection of an imminent relapse (Bruggemann et al., SemOncol 2012). Allele-specific oligonucleotide (ASO)-PCR can be used to assess MRD; however, this technique requires preparation of clonotype-specific primers for each individual which is laborious and time-consuming. We recently demonstrated the utility of sequencing-based MRD assessment in lymphoid malignancies (Faham et al., Blood 2012). This quantitative approach, termed the LymphoSIGHT™ platform, relies on amplification and sequencing of immunoglobulin and T-cell receptor gene segments using consensus primers and can address some of the limitations associated with traditional MRD detection techniques. This sequencing platform has a sensitivity to detect one cancer cell per million leukocytes in peripheral blood and bone marrow samples. In this retrospective study, we evaluated the ability of the sequencing and ASO-PCR methods to detect MRD prior to clinical relapse in 17 patients with childhood ALL.
Using the sequencing assay, we analyzed bone marrow and/or peripheral blood samples collected at the diagnostic and relapse time points from 17 childhood ALL patients. Diagnostic and relapse samples were assessed for clonal rearrangements of immunoglobulin (IGH-VDJ, IGH-DJ, IGK) and T cell receptor (TRB, TRD, TRG) genes. Following identification of leukemia specific clonotype(s), we measured the corresponding MRD levels in 66 follow-up samples that were collected prior to clinical relapse. We analyzed the time from MRD positivity to clinical relapse for each patient using the sequencing and ASO-PCR methods.
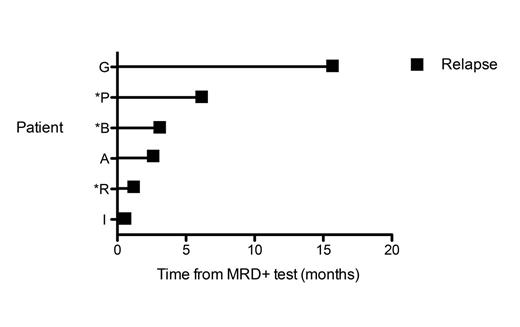
Sequencing detected the presence of a high-frequency clonal rearrangement of at least one receptor (“calibrating receptor”) in all the 17 childhood ALL patients; all patients had at least 2 calibrating receptors at diagnosis and/or relapse, 15 patients had at least 3 calibrating receptors, and 6 patients had 4 or more. The IGH-VDJ and TRG assays were the most frequent gene rearrangement: at least one IGH-VDJ and/or TRG clonal rearrangement was detected in 13 ALL patients. TRD was the third most informative receptor, with clonal rearrangements being detected in 11 patients. In the majority of patients, most leukemic clones identified at diagnosis were also present at high levels in the relapse sample. We analyzed the time from MRD positivity to clinical relapse in the patient cohort. In 2 patients (Figure 1, Patients G and P), we observed MRD positivity by sequencing 16 and 6.5 months prior to relapse, respectively. ASO-PCR also detected MRD positivity in Patient P at the 6.5 month time point. In 6 patients there was a sample collected within 1-3 months prior to relapse, and sequencing detected MRD in 4 of these patients (67%, Figure 1 Patients B, A, R, I), while ASO-PCR detected MRD in 2 of the 6 patients (33%, Figure 1 Patients B, R).
The clinical value of monitoring MRD by ASO-PCR for assessment of treatment response and outcome has been established in multiple lymphoid malignancies. This preliminary study provides further support for the sequencing-based MRD monitoring in ALL patients. This new approach offers improvements over ASO-PCR in the ability to monitor multiple clonal sequences and in the sensitivity to detect the leukemic cell. Thus, the sequencing method represents a new potential approach for MRD monitoring. However a larger number of patients must be analyzed to support its clinical application.
Time elapsed from a sequencing-based MRD positive test to clinical relapse in ALL patients. All six patients included in the figure had a positive sequencing MRD test prior to relapse. Three patients denoted by an asterisk were MRD positive by both sequencing and ASO-PCR at the same time point prior to relapse.
Time elapsed from a sequencing-based MRD positive test to clinical relapse in ALL patients. All six patients included in the figure had a positive sequencing MRD test prior to relapse. Three patients denoted by an asterisk were MRD positive by both sequencing and ASO-PCR at the same time point prior to relapse.
Faham:Sequenta, Inc.: Employment, Equity Ownership, Membership on an entity’s Board of Directors or advisory committees. Pepin:Sequenta, Inc.: Employment, Equity Ownership. Carlton:Sequenta, Inc.: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.