Abstract
The identification of mutations (mut) in SETBP1 recently shed light on a molecular marker in atypical chronic myeloid leukemia (aCML), a disease previously defined by exclusion criteria. SETBP1mut have been identified in different myeloid malignancies. We previously reported mutation frequencies in the range of 5-10% in MPN and MDS/MPN overlap, 32% in aCML, while we found SETBP1 less frequently mutated in AML (3%). SETBP1mut has been shown to associate with ASXL1, CBL and SRSF2 mutations, as well as the cytogenetic abnormalities -7 and i(17)(q10).
To investigate the mutation frequency of ASXL1, SETBP1, and SRSF2 in different myeloid entities in correlation to the cytogenetic abnormalities -7 and i(17)(q10).
A cohort of 451 patients (pts) with different myeloid entities was analyzed. Diagnoses according to cytomorphology followed the WHO classification from 2008 (n=439, for n=12 cases no cytomorphology was available): AML (n=29), aCML (n=62), MDS/MPN overlap (n=16), CMML (n=283), MDS (n=5), MPN (n=43), CML (n=1). The cohort consisted of 303 males and 148 females; cytogenetics was available in 445 cases. Patients were grouped by normal karyotype (n=291), i(17)(q10) (n=16), -7 (n=22), and other cytogenetic aberrations (n=117); one case carried both a i(17)(q10) and a -7. ASXL1 exon 13, the mutational hotspot regions of SETBP1 and SRSF2 were analyzed by Sanger sequencing in all cases.
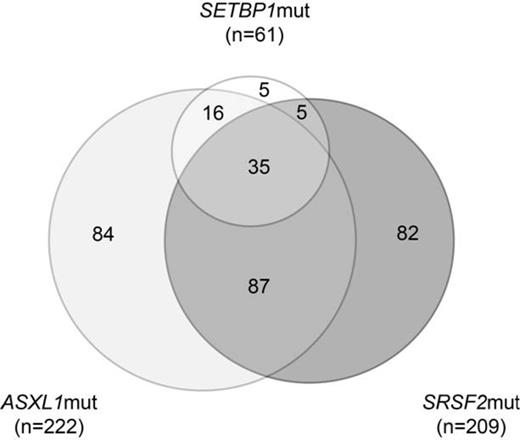
In the total cohort ASXL1 was mutated in 222/451 (49%), SETBP1 in 61/451 (14%), and SRSF2 in 209/451 (46%) cases. 137 pts showed no mutation in any of these three genes. 171 pts carried one mutation, thereof 84 a sole ASXL1mut, 82 a sole SRSF2mut and only 5 cases showed sole SETBP1mut. In 108 pts two, and in 35 pts all three analyzed genes were mutated. The most frequent combination within the group with two mutations was ASXL1 and SRSF2 (n=78), followed by ASXL1 and SETBP1 (n=16), only 5 cases were mutated in SRSF2 and SETBP1. Addressing the association with cytogenetics revealed that in cases with only one mutation SRSF2mut associated as sole mutation with a normal karyotype (68/124 (55%) SRSF2mut in the normal karyotype group vs. 12/42 (28%) SRSF2mut in all other karyotypes; p=0.003). In contrast, ASXL1mut and SETBP1mut as sole mutations showed no correlation to any addressed karyotype. However, addressing the cases with two mutations the combination of SRSF2mut and ASXL1mut correlated with a normal karyotype (67/291 (23%) SRSF2mut/ASXL1mut in the normal karyotype group vs. 19/154 (12%) SRSF2mut/ASXL1mut in all other karyotypes; p=0.008), while SRSF2mut and SETBP1mut occurred more frequently in i(17)(q10) pts (2/16 (13%) SRSF2mut/SETBP1mut in i(17)(q10) vs. 2/429 (1%) SRSF2mut/SETBP1mut in all other karyotypes; p=0.007). Remarkably, cases with mutations in all three analyzed genes (ASXL1mut, SETBP1mut, and SRSF2mut) highly associated with i(17)(q10) and -7. 11 of 16 cases with i(17)(q10) (69%) showed all three mutations (vs. 24/429 (6%) in all other karyotypes; p<0.001). Furthermore, 6 of 22 cases with -7 (27%) showed mutations in all three genes (vs. 29/423 (7%); p=0.005). Therefore, 15 pts carried all three mutated genes as well as i(17)(q10) or -7. Interestingly, there was no case with only i(17)(q10) and no additional mutation, and only one case with i(17)(q10) and only one additional molecular mutation, 4 cases with two additional molecular mutations and 11 cases carrying all three mutations, possibly indicating that i(17)(q10) appear during clonal evolution. Therefore one might assume that this represents a specific genetic phenotype that is driven by the accumulation of molecular events, since addition of SETBP1mut shifts the association from a normal karyotype to i(17)(q10) or -7. Analyzing the distribution of these cases for mutations in all three analyzed genes +/- additional cytogenetic aberration i(17)(q10) or -7 in the different myeloid entities showed that AML, aCML, CMML, MDS as well as MPN showed this genetic phenotype (AML: n=7 (24%); atypical CML: n=12 (19%); CMML: n=8 (3%); MDS: n=1 (20%); MPN: n=6 (14%)).
Mutations in SETBP1 associate with ASXL1mut and SRSF2mut and are frequently found in patients with i(17)(q10) or -7. This combination of genetic lesions occurs in different myeloid entities and might therefore define a specific genetically defined subtype of myeloid malignancy.
Meggendorfer:MLL Munich Leukemia Laboratory: Employment. Alpermann:MLL Munich Leukemia Laboratory: Employment. Haferlach:MLL Munich Leukemia Laboratory: Employment, Equity Ownership. Sirch:MLL Munich Leukemia Laboratory: Employment. Kern:MLL Munich Leukemia Laboratory: Employment, Equity Ownership. Haferlach:MLL Munich Leukemia Laboratory: Employment, Equity Ownership. Schnittger:MLL Munich Leukemia Laboratory: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

