Key Points
PECAM-1 deficiency misguides venous thrombus resolution.
PECAM-1 cell surface shedding occurs at the site of venous thrombosis.
Abstract
Platelet endothelial cell adhesion molecule 1 (PECAM-1) is involved in leukocyte migration and angiogenesis, which are key components of venous thrombus resolution. This study investigated the effect of PECAM-1 deficiency on thrombus resolution in FVB/n mice and the extent to which levels of soluble PECAM-1 (sPECAM-1) correlate with delayed thrombus resolution in humans after acute symptomatic deep vein thrombosis (DVT). In a mouse stagnant flow venous thrombosis model Pecam-1−/− thrombi were larger, persisted for longer periods of time, and displayed attenuated macrophage invasion and decreased vessel formation in the presence of increased fibrosis. In humans, higher levels of truncated plasma sPECAM-1 possibly cleaved from cell surfaces, were found in patients with delayed thrombus resolution (assessed via duplex-based thrombus scoring) relative to those whose thrombi resolved (median, 25th/75th percentile): 92.5 (87.7/103.4) ng/mL vs 71.5 (51.1/81.0) ng/mL; P < .001. Furthermore, unresolved human deep vein thrombus specimens stained positively with antibodies specific for the extracellular, but not the cytoplasmic domain of PECAM-1, consistent with accumulation of cleaved PECAM-1. Our data suggest a regulatory role of PECAM-1 in venous thrombus resolution and suggest a predictive value of sPECAM-1 for postthrombotic syndrome (PTS) after acute DVT.
Introduction
Venous thromboembolism (VTE) (ie, deep-vein thrombosis [DVT] or pulmonary embolism) is a common disorder with an annual incidence of approximately 1 or 2 cases per 1000 persons in the general population.1 Short-term treatment is effective, but long-term complications clinically manifest as postthrombotic syndrome (PTS) or chronic thromboembolic pulmonary hypertension (CTEPH). Although CTEPH is rare,2 1 of 2 to 3 patients with DVT will develop PTS within 2 years,3 which leads to significant socioeconomic consequences due to high morbidity and treatment costs. Clinical manifestations of PTS are caused by increased hydrostatic pressure due to venous valve dysfunction and persistent thrombus, and range from chronic pain, heaviness, itching, edema, and cramping to ulcers in the affected leg. Treatment is limited to palliation with mechanical leg compression and symptomatic ulcer care.
Cellular mechanisms of impaired venous thrombus resolution remain unclear. A transient proinflammatory status is regarded to be beneficial for thrombus clearance, but might contribute to the damage of vessel wall structures. Physiological resolution comprises reorganization of the extracellular matrix at the site of thrombosis via fibrinolysis and angiogenesis. Innate immune cells coordinate these mechanisms as neutrophil granulocytes are the first to invade a newly formed thrombus and promote fibrinolysis and collagenolysis.4 Consequently, neutropenia is associated with larger venous thrombi and increased fibrosis in various animal models.5 Subsequently, neutrophil granulocytes are replaced by monocytes. Proinflammatory signaling molecules, such as monocyte chemotactic protein 16 and IL-8,7 stimulate their recruitment and accelerate thrombus resolution in vivo. Leukocyte migration to the site of inflammation and thrombosis is not fully understood, but is thought to be mediated by cell adhesion molecules, one of them being platelet endothelial cell adhesion molecule 1 (PECAM-1).8-10
PECAM-1 is a single chain glycopeptide receptor of 130 kDa relative molecular weight and is expressed on platelets, endothelial cells, macrophages, neutrophils, lymphocytes, and bone marrow cells.11 The cytoplasmatic domain of the molecule participates in intracellular signaling cascades via the immunoreceptor tyrosine-based inhibitory motif.12 PECAM-1 is involved in leukocyte transmigration, regulation of leukocyte responses to inflammatory stimuli,13 and vascular development,14-17 which are major determinants of venous thrombus resolution.18 Soluble PECAM-1 (sPECAM-1) is generated either by PECAM-1 proteolytic cleavage at the cell surface or by alternative splicing upon cell activation.19,20 Hence, sPECAM-1 exists in at least 2 isoforms: A truncated form that lacks the cytoplasmic tail and results from cell surface shedding, and a transmembrane-less form that comprises both the extracellular and cytoplasmic domains and originates from splicing out the transmembrane region.
The aim of our study was to elucidate the role of PECAM-1 in venous thrombus resolution. We hypothesized that PECAM-1 deficiency impacts leukocyte thrombus invasion and angiogenesis, and consequently misguides thrombus resolution.
Materials and methods
Murine model of stagnant flow venous thrombosis
Wild-type and Pecam-1−/− mice in the FVB/n background were housed at the animal facility of the Medical University of Vienna (protocol approved by the Austrian Ministry of Science). Pecam-1−/− mice were generated as previously described by Schenkel et al,21 and were obtained from William A. Muller (Department of Pathology, Medical College of Cornell University, New York, NY). PECAM-1 deficient mice do not exhibit a general defect in leukocyte trafficking. The requirement of PECAM-1 in this process is rather stimulus-dependent10 and strain-specific. In contrast to the C57BL/6 Pecam-1−/− strain, the inbred FVB/n Pecam-1−/− strain is not able to compensate for the loss of PECAM-1 regarding leukocyte function,22 and was therefore chosen for the experiments.
In the murine model, thrombi were induced in the infrarenal vena cava of narcotized mice (n = 110; 8- to 12-week-old animals weighing 21 to 32 g were given 100 mg/kg ketamine and 5 mg/kg xylazine intraperitoneally) via midline laparotomy incision. A 5-0 suture (Prolene) was placed alongside the vena cava and a stenosis was produced in the vein by tying a 4-0 silk suture around the vena cava to include the Prolene. The Prolene was then pulled to allow blood to continue to pass up the vein. The abdominal wall was sutured and infrarenal venous thrombi of both groups were subsequently excised and prepared for analysis at fixed time intervals (days 3, 7, 14, and 28) after ligation. Tissues were fixed in 7.5% buffered formaldehyde, 3 μm paraffin sections were prepared for (immuno)histochemical analysis. For thrombus area quantification an Olympus BX 50 microscope equipped with the imaging software Axio (Version 3.0; Carl Zeiss Vision GmbH, Germany) was used. Thrombus lengths, cross-sectional areas, and volumes (lengths × cross-sectional areas) were determined on days 3, 7, 14, and 28 after vena cava ligation. Mean relative volume changes were calculated as the differences of mean thrombus volumes between 2 subsequent time points.
Modified trichrome stain
For histological analysis, a modified trichrome stain was used, as previously described.23 Both early fibrin and red blood cells are shown by Lissamine Fast Yellow. Mature fibrin is stained by a combination of acid fuchsin, biebrich scarlet and ponceau 2R (red), whereas collagen is visualized by a green color.
Immunohistochemistry
Immunohistochemical analysis was performed as previously described.24 For the labeled-streptavidin-biotin technique, we used a Histostain SP kit (95-6543 AEC Mouse Kit; Zymed Laboratories Inc., San Francisco, CA). Cell characterization was performed using the following primary antibodies: anti-murine F4/80 glycoprotein antibody (14-4801, Ebioscience), anti-murine neutrophil monoclonal antibody (CL8993AP; Cedarlane Laboratories), monoclonal anti-murine α-smooth muscle actin antibody (M851; DAKO), and monoclonal anti-murine isolectin B4 antibody (B-1205; Vector Laboratories).
Immunohistochemical staining with domain-specific antibodies for the intracellular portion (iPECAM-1; mAb 235.1) and the extracellular domain (ePECAM-1; anti CD31 M0823; DAKO) of human PECAM-1 was performed using Histostain-SP Broad Spectrum Kit (959943 AEC; Invitrogen), according to the manufacturer’s instructions. Cleaved PECAM-1 is visualized by N-terminal PECAM-1 (M0823) immunoreactivity in the absence of C-terminal PECAM-1 (mAb 235.1) immunoreactivity.
Measurement of collagen concentration
Collagen concentration in paraffin-embedded sections was quantified as previously described.25 The method is based on the selective binding of Sirius Red F3BA and Fast Green FCF to collagen and noncollagenous proteins. The dyes have maximum absorbances at different wavelengths. One mL of 0.1 M NAOH in absolute methanol is added to elute the color from the sections. Eluted dye is read in a spectrophotometer (Nano Drop ND 1000) at 540 and 605 nm. Collagen content is expressed as collagen in percent of total protein content.
Real-time PCR
Total RNA was extracted from murine/human tissue using RNeasy Mini Kit and RNeasy Fibrous Tissue Kit (Qiagen). Complementary DNA was synthesized from 2μg of total RNA by reverse transcription (TaqMan Reverse Transcription Reagents, 8080234; Invitrogen). Quantitative fluorogenic real-time PCR was performed in an ABI PRISM 7000 Sequence Detector (Applied Biosystems, Foster City, CA). Specific TaqMan primers and probes for murine common leukocyte marker CD68 (ID: Mm00839636_g1) and human PECAM-1 (ID: Hs00169777_ml) were used (TaqMan Gene Expression Assay, Applied Biosystems). Messenger RNA (mRNA) expression level was normalized to endogenous eukaryotic 18S ribosomal RNA levels. Results are expressed as ratios of specific gene expression.
Study population and procedures
Consecutive patients (n = 48; whites) with a diagnosis of acute, symptomatic popliteal or proximal DVT were enrolled after providing written informed consent in accordance with the Declaration of Helsinki under a protocol reviewed by the ethics committee of the General Elisabethinen Hospital, Linz, Austria. Exclusion criteria were an expected life span less than 1 year and coexisting symptomatic pulmonary embolism (assessed either via computer tomography pulmonary angiography or standard 6-view perfusion lung scan).
Thrombus score (reflecting the burden of leg vein thrombus) was calculated using manual complete compression ultrasonography.26 The score for each affected venous segment (except for the deep femoral) was adjusted for thrombus diameter (assessed under full compression and compared with the diameter of the corresponding artery). The segment score was increased 1.5-fold if its compressed diameter was ≥1.5 times the arterial diameter, and reduced 0.5-fold if this was ≤0.5 times the arterial diameter. The thrombus score was calculated as the sum of each segment: external iliac vein (8), common femoral vein (4), deep femoral vein (2), proximal (4) and distal (3) superficial femoral vein, popliteal vein (2), posterior tibial veins (2), and peroneal veins (2). A score ≥4 at baseline was an inclusion criterion.
At follow-up, a duplex scan was scheduled for 28 (±3) days after diagnosis to evaluate residual vein thrombus and to calculate change of thrombus score (Δ = thrombus score baseline-thrombus score on day 28). A Δ thrombus score <4 was defined as delayed thrombus resolution. All patients received compression stockings, a therapeutic dose of low-molecular-weight heparin and/or subsequent oral anticoagulation therapy. Adequate oral anticoagulation therapy therapy was defined by an international normalized ratio of 2 to 3.
Blood samples for sPECAM-1 enzyme linked immunosorbent assay (ELISA) were collected within 24 hours after diagnosis and at follow-up appointment and were stored at −80°C until final analysis. After 12 (±0.5) months patients were evaluated for PTS using the Villalta scale: points from 0 (absent) to 3 (severe) for 5 patient-rated symptoms (pain, cramps, paresthesia, pruritus, heaviness) and 6 clinician-rated symptoms (pretibial edema, redness, skin induration, hyperpigmentation, venous ectasia, pain during calf compression) were summed into a total score (range, 0-33). PTS was defined by a Villalta score ≥5. Investigators performing duplex scan and PTS evaluation did not have access to the results of blood analyses at any time.
sPECAM-1/soluble intercellular adhesion molecule 1 sandwich ELISA
Total sPECAM-1 measurements were performed utilizing indirect enzyme-linked immunosorbent assay (Platinum ELISA Bender MedSystems BMS 229), according to the manufacturer’s instructions. The monoclonal anti-human mouse antibody directed against the cytoplasmic tail of PECAM-1 (235.1 provided by Peter J. Newman, PhD, Blood Research Center of Wisconsin, Milwaukee, WI) was used to detect the soluble subform comprising the cytoplasmic domain: the IgG antibody was diluted in HEPES to 25 μg/mL and adsorbed onto microtiter plates overnight (4°C). Wells were rinsed (phosphate-buffered saline 0.05% Tween 20) and blocked with 1% bovine serum albumin for 1 hour at room temperature, followed by a 1-hour incubation of the plasma sample. Horseradish peroxidase -conjugated anti-human sPECAM-1 monoclonal detection antibody directed against the extracellular domain was added and incubated for 3 hours at room temperature. Microwells were rinsed and color was developed by addition of a substrate solution (tetramethylbenzidine). Absorbances were read on a spectrophotometer using 450 nm as the primary wavelength after 10 minutes.
For soluble intercellular adhesion molecule 1 (sICAM-1) determination, we used the sICAM-1 Platinum ELISA (Ebioscience BMS 201) according to the manufacturer’s instructions.
Human tissue samples
Human tissue samples of nonresolving DVT thrombi (n = 4) and venous vessel wall (n = 4) that were harvested in the course of variceal surgeries were fixed in 7.5% formaldehyde for immunohistochemistry. Pulmonary endarterectomy specimens from patients with CTEPH (n = 6), and nonthrombosed pulmonary arterial vessel wall (pulmonary artery, n = 4) were harvested during respective surgeries and were immediately snap frozen in liquid nitrogen for real-time PCR.
Statistics
The SPSS statistical package (version 18.0.2) was used. Murine data were expressed as means and standard deviations, and significance of intergroup differences was examined using the Student’s unpaired t test. Levels of sPECAM-1, sICAM-1, and D-Dimer were described by medians and interquartile-ranges (25th percentiles/75th percentiles) with an intergroup (Δ thrombus score <4 vs ≥4, PTS yes vs no) difference determined by the Mann-Whitney U test. Differences regarding PTS status in patients with normal and delayed thrombus resolution were determined using Fisher’s exact test. Significance of intergroup gene expression differences was determined by analysis of variance. P < .05 was considered statistically significant. Univariate logistic regression was used to determine the predictive value of baseline sPECAM-1. Odds ratios (OR) and risk ratios are provided with 95% confidence intervals and P values. To identify potential confounders, we simultaneously added baseline patient characteristics to the univariate model to calculate adjusted OR. Correlation between sPECAM-1 levels and Δ thrombus score was determined using Bravais-Pearson’s correlation coefficient. For the correlation between sPECAM-1 levels and D-Dimer levels, the Spearman’s ρ was used.
Results
Pecam-1−/− venous thrombi are larger, more persistent, and characterized by less inflammatory cells and fewer vessels
We were able to harvest thrombi in 96% of Pecam-1−/− mice and in 84% of controls resulting in 110 thrombi (n = 8 to 20 per each group and individual time point).
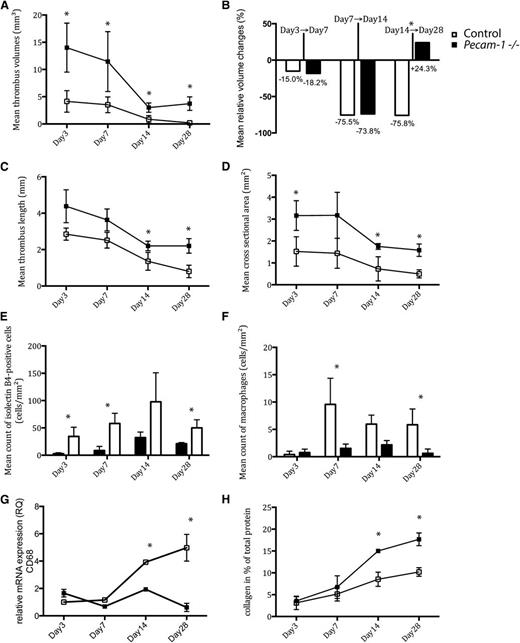
Quantitative thrombus analysis revealed that Pecam-1−/− mice exhibited significantly larger thrombi on days 0 to 28 (Figure 1A,C-D). Mean thrombus volume peaked by day 3 and regressed to its minimum value by day 14. In contrast to controls, Pecam-1−/− mice showed overall impaired thrombus resolution with a period of thrombus length (Figure 1C) and volume increase (Figure 1A) between days 14 and 28. Mean relative thrombus volume changes are illustrated in Figure 1B. By day 28, the mean cross-sectional area of Pecam-1−/− thrombi was nearly fourfold that of controls (1.5 ± 0.2 mm2 vs 0.4 ± 0.2 mm2; P < .001) (Figure 1D).
Temporal changes of thrombus characteristics in Pecam-1−/−and wild-type mice. Thrombus volumes (A), relative thrombus volume changes (B), thrombus lengths (C), cross-sectional areas (D), endothelial cell counts (E), macrophage counts (F), relative CD68 mRNA expression levels (G), and collagen deposition (H) on days 3, 7, 14, and 28 after vena cava ligation are shown (*P < .05, all means with ± standard deviations). Black symbols represent Pecam-1−/− mice; white symbols represent controls.
Temporal changes of thrombus characteristics in Pecam-1−/−and wild-type mice. Thrombus volumes (A), relative thrombus volume changes (B), thrombus lengths (C), cross-sectional areas (D), endothelial cell counts (E), macrophage counts (F), relative CD68 mRNA expression levels (G), and collagen deposition (H) on days 3, 7, 14, and 28 after vena cava ligation are shown (*P < .05, all means with ± standard deviations). Black symbols represent Pecam-1−/− mice; white symbols represent controls.
Staining characteristics indicated that Pecam-1−/− thrombi (Figure 2) contained significantly larger areas of collagen compared with controls on day 28 (17.6 ± 1% vs 10.2 ± 1%; P < .001) (Figure 1H). Furthermore, Pecam-1−/− macrophages were less frequently found in thrombi (Figure 1F and Figure 3M-P); (day 7: 9.5 ± 4.7 cells/mm2 vs 1.5 ± 0.7 cells/mm2; P = 0,019/day 28: 5.8 ± 2.9 cells/mm2 vs 0.6 ± 0.8 cells/mm2; P = .013). Accordingly, expression of the leukocyte marker CD68 was reduced at the site of thrombosis in Pecam-1−/− mice (Figure 1G). By contrast, numbers of smooth muscle cells and neutrophils (data not shown) were comparable to those in controls at all time points.
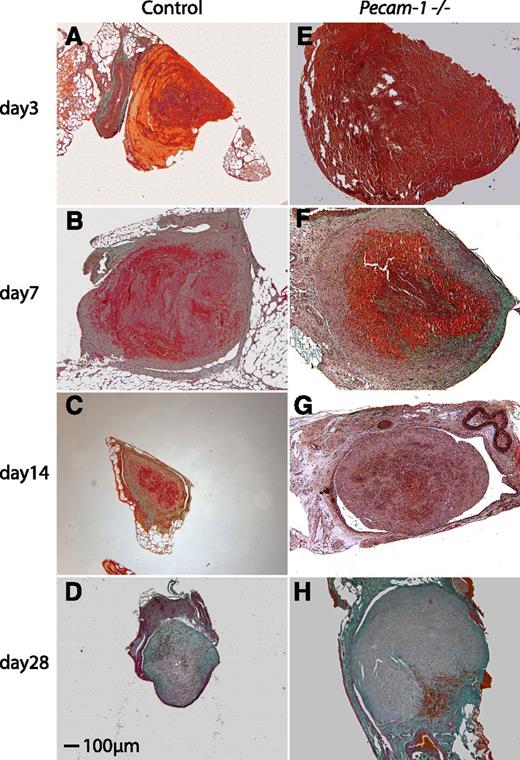
Trichrome stains of wild-type and Pecam-1−/−thrombi. Representative thrombus cross-sections of wild-type (A-D) and Pecam-1−/− (E-H) mice on days 3 (A,E), 7 (B,F), 14 (C,G), and 28 (D,H) after vena cava ligation are shown. Initially, thrombi are fibrin-rich (red) and fragile, and subsequently become solid due to increased collagen synthesis (green). Scale bar, 100 μm.
Trichrome stains of wild-type and Pecam-1−/−thrombi. Representative thrombus cross-sections of wild-type (A-D) and Pecam-1−/− (E-H) mice on days 3 (A,E), 7 (B,F), 14 (C,G), and 28 (D,H) after vena cava ligation are shown. Initially, thrombi are fibrin-rich (red) and fragile, and subsequently become solid due to increased collagen synthesis (green). Scale bar, 100 μm.
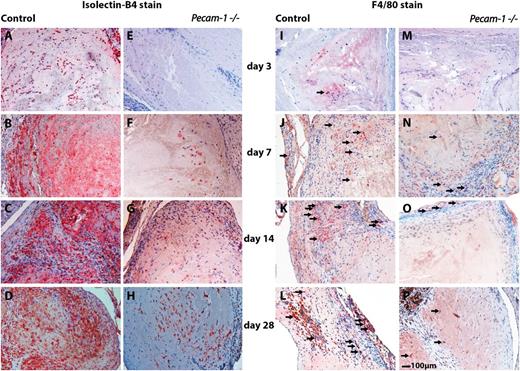
Immunohistochemical analyses of wild-type and Pecam-1−/−thrombi illustrating misguided vascular remodeling of thrombus resolution in the absence of PECAM-1. Isolectin-B4 immunoreactivities representing endothelial cells in wild-type (A-D) and corresponding Pecam-1−/− thrombi (E-H) are shown on days 3 (A,E), 7 (B,F), 14 (C,G), and 28 (D,H) after vena cava ligation on the left side of the panel. The right side of the panel shows F4/80 immunoreactivities representing macrophages (examples of immunopositive cells are pointed out with small arrows) in wild-type (I-L) and corresponding Pecam-1−/− thrombi (M-P) on days 3 (I,M), 7 (J,N), 14 (K,O), and 28 (L,P) after vena cava ligation. Scale bar, 100 μm.
Immunohistochemical analyses of wild-type and Pecam-1−/−thrombi illustrating misguided vascular remodeling of thrombus resolution in the absence of PECAM-1. Isolectin-B4 immunoreactivities representing endothelial cells in wild-type (A-D) and corresponding Pecam-1−/− thrombi (E-H) are shown on days 3 (A,E), 7 (B,F), 14 (C,G), and 28 (D,H) after vena cava ligation on the left side of the panel. The right side of the panel shows F4/80 immunoreactivities representing macrophages (examples of immunopositive cells are pointed out with small arrows) in wild-type (I-L) and corresponding Pecam-1−/− thrombi (M-P) on days 3 (I,M), 7 (J,N), 14 (K,O), and 28 (L,P) after vena cava ligation. Scale bar, 100 μm.
Finally, Pecam-1−/− thrombi were characterized by diminished thrombus vessels (Figure 1E and Figure 3A-H). Newly formed microvessels were detectable in the periphery of control thrombi by day 3 (34.3 ± 16.9 isolectin B4-positive cells/mm2 in control thrombi vs 2.7 ± 1.8 isolectin B4-positive cells/mm2 in Pecam-1−/− thrombi; P = .014), whereas Pecam-1−/− thrombi showed less vascularization until day 14 (day 7: 58.0 ± 18.6 cells/mm2 vs 8.5 ± 7.4 cells/mm2; P = .01/day 14: 97.8 ± 53.0 cells/mm2 vs 32.3 ± 10.1 cells/mm2; P = .87).
Serum of patients with delayed thrombus resolution contains elevated sPECAM-1 levels at baseline
Characteristics and results of patients with acute, symptomatic DVT who completed 1 year of follow-up are shown in Table 1. Right-sided DVT was diagnosed in 46.9%, left-sided DVT in 46.9%, and bilateral DVT in 6.2% of all patients. Malignancy was observed in 27.1% of patients and a previous episode of VTE in 39.6%. Duration of anticoagulation therapy ranged between 3 months and indefinitely, depending on individual risk of recurrence. There were 45.8% of all patients who displayed delayed thrombus resolution 1 month after diagnosis. Cumulative incidence of PTS in 46 patients after 1 year was 32.6%.
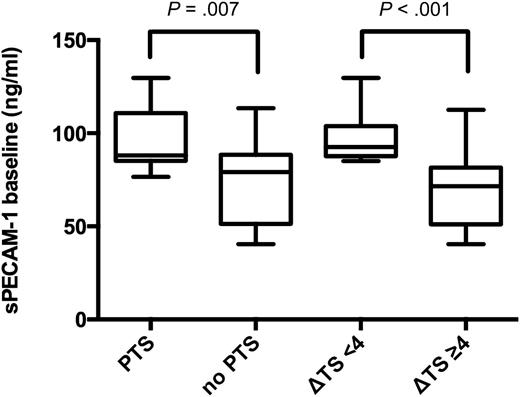
In 16.7% of patients with Δ thrombus score ≥4, PTS developed within 1 year. These patients had median (25th/75th percentile) sPECAM-1 baseline plasma levels of 71.5 (51.1/81.0) ng/mL and median D-Dimer baseline plasma levels of 4.5 (3.1/11.0) μg/mL (Table 1). Patients with Δ thrombus score <4, however, developed PTS in 50% of cases [risk ratio of 3.00 (95% CI 1.108-8.124; P = .002)], and showed median sPECAM-1 baseline plasma levels of 92.5 (87.7/103.4) ng/mL (P < .001), and median D-Dimer baseline plasma levels of 3.3 (2.1/4.4) μg/mL (P = .069) (Table 1 and Figure 4). On follow-up median sPECAM-1 levels were 90.0 (73.0/102.6) ng/mL vs 71.9 (57.2/87.0) ng/mL (P = .049) in patients with Δ thrombus score <4 vs ≥4 (Table 1). Pearson’s coefficient for correlation between Δ thrombus score and sPECAM-1 baseline levels was −0.467 (P = .001); Spearman’s ρ for correlation between sPECAM-1 baseline levels and baseline D-Dimer levels was −0.326 (P = .027).
Baseline sPECAM-1 plasma levels (ng/mL) in study patients. Box plots illustrating baseline sPECAM-1 levels (ng/mL) in patients with subsequent PTS vs patients without PTS (shown on the left side of the figure; P = .007). The 2 box plots on the right side of the figure show sPECAM-1 levels (ng/mL) in patients with subsequently delayed thrombus resolution (Δ thrombus score <4), compared with patients undergoing normal thrombus resolution (Δ thrombus score ≥4; P < .001).
Baseline sPECAM-1 plasma levels (ng/mL) in study patients. Box plots illustrating baseline sPECAM-1 levels (ng/mL) in patients with subsequent PTS vs patients without PTS (shown on the left side of the figure; P = .007). The 2 box plots on the right side of the figure show sPECAM-1 levels (ng/mL) in patients with subsequently delayed thrombus resolution (Δ thrombus score <4), compared with patients undergoing normal thrombus resolution (Δ thrombus score ≥4; P < .001).
Baseline sPECAM-1 levels predicted thrombus persistence [OR of 1.134 (95% CI 1.046-1.229; P = .002)] both in univariate logistic regression analysis and multivariate logistic regression analysis corrected for age, gender, and malignancy [adjusted OR of 1.139 (95% CI 1.051-1.235; P = .001)]. In addition, baseline sPECAM-1 plasma levels were predictive of PTS (adjusted OR of 1.350 [95% confidence interval, 1.049-1.739; P = .020]). Baseline soluble ICAM-1 plasma levels did not significantly differ between patients with subsequent PTS and those who did not develop PTS (565.8 [480.1/771.3] ng/mL vs 499.1 [466.2/589.3] ng/mL; P = .210), however, levels tended to be higher in the PTS group, which has been described.27
Patients with PTS showed a median Villalta score of 6.0 (5.0/7.0) and median baseline sPECAM-1 levels of 88.0 (85.2/110.2) ng/mL compared with median Villalta score of 2.0 (1.0/4.0) and median baseline sPECAM-1 levels of 79.2 (51.5/88.2) ng/mL (P = .007) in patients without PTS (Figure 4).
Total thrombus burden expressed by the most proximal thrombus edge (Table 1) was not significantly different between the groups.
Use of antibody 235.1 directed against the cytoplasmic domain as coating antibody in the ELISA assays failed to detect sPECAM-1 in patients with Δ thrombus score <4 and in patients with Δ thrombus score ≥4, suggesting that sPECAM-1 levels in our patients exclusively represented the truncated form.
Immunohistochemistry with domain-specific antibodies suggests PECAM-1 shedding at the site of venous thrombosis
The truncated form of sPECAM-1 results from proteolytic cell surface cleavage. To identify the source of elevated truncated sPECAM-1 plasma levels in patients with delayed thrombus resolution, we analyzed human nonresolving DVT tissue specimens in comparison with human unthrombosed saphenous vein vessel wall specimens. We used domain-specific PECAM-1 antibodies directed against ePECAM-1 (N-terminal) and iPECAM-1 (C-terminal) to determine the presence of both domains indicating expression of full-length PECAM-1. In unthrombosed venous vessel sections, immunoreactivity for the extracellular and intracellular domain of PECAM-1 was found, suggesting the presence of full-length PECAM-1 (Figure 5C2,C3, arrows), whereas nonresolving deep vein thrombi stained positively with antibodies specific for ePECAM-1 (Figure 5A2,B2), but not iPECAM-1 (Figure 5A3,B3), suggesting cleaved forms of PECAM-1. A discordance of extracellular and intracellular PECAM-1 domain expression (Figure 5A3,B3, asterisk) was previously interpreted as an accumulation of truncated forms of PECAM-1.28
Differential domain-specific antibody immunoreactivities suggesting PECAM-1 cell surface shedding at the site of thrombosis. The left side of the panel shows trichrome stains of 2 cases of nonresolving human deep vein thrombus (specimen A-B, A1, and B1) and the vessel wall from a human saphenous vein (C1). These tissue samples were immunhistochemically stained using domain-specific antibodies directed against the extracellular domain (N-terminal) of PECAM-1 (ePECAM-1: A2, B2, and C2) and intracellular portion (C-terminal) of PECAM-1 (iPECAM-1: A3, B3, and C3). Cleaved PECAM-1 is characterized by the presence of immunoreactivity derived from the antibody against ePECAM-1 in the absence of immunoreactivity derived from the antibody against iPECAM-1. The area of discordant PECAM-1 expression (A2 vs A3; B2 vs B3) is highlighted with an asterisk (A3, B3). By contrast, the endothelial cell layer of saphenous vein wall stains positively with both antibodies, indicating full length PECAM-1 expression (C2-3, arrows). Scale bar, 100 μm.
Differential domain-specific antibody immunoreactivities suggesting PECAM-1 cell surface shedding at the site of thrombosis. The left side of the panel shows trichrome stains of 2 cases of nonresolving human deep vein thrombus (specimen A-B, A1, and B1) and the vessel wall from a human saphenous vein (C1). These tissue samples were immunhistochemically stained using domain-specific antibodies directed against the extracellular domain (N-terminal) of PECAM-1 (ePECAM-1: A2, B2, and C2) and intracellular portion (C-terminal) of PECAM-1 (iPECAM-1: A3, B3, and C3). Cleaved PECAM-1 is characterized by the presence of immunoreactivity derived from the antibody against ePECAM-1 in the absence of immunoreactivity derived from the antibody against iPECAM-1. The area of discordant PECAM-1 expression (A2 vs A3; B2 vs B3) is highlighted with an asterisk (A3, B3). By contrast, the endothelial cell layer of saphenous vein wall stains positively with both antibodies, indicating full length PECAM-1 expression (C2-3, arrows). Scale bar, 100 μm.
Gene expression analysis demonstrates diminished PECAM-1 expression in chronic venous thrombosis
Analysis of PECAM-1 expression in vascular tissues confirmed significant downregulation of PECAM-1 in nonresolving thrombi compared with nonthrombosed pulmonary artery (Figure 6) (P = .009). Endarterectomy specimens from patients with CTEPH were used as extreme examples for persistent/recurrent thrombosis.
Low relative expression of PECAM-1 mRNA within nonresolving human thrombi. The figure shows real-time PCR analysis of CTEPH thrombi (n = 6) and nonresolving DVT thrombi (n = 3) compared with pulmonary artery (n = 4). Chronic nonresolving thrombi are characterized by significant downregulation of PECAM-1 mRNA (normalized to 18S ribosomal RNA). PA, pulmonary artery.
Low relative expression of PECAM-1 mRNA within nonresolving human thrombi. The figure shows real-time PCR analysis of CTEPH thrombi (n = 6) and nonresolving DVT thrombi (n = 3) compared with pulmonary artery (n = 4). Chronic nonresolving thrombi are characterized by significant downregulation of PECAM-1 mRNA (normalized to 18S ribosomal RNA). PA, pulmonary artery.
Discussion
Current data suggest that physiological venous thrombus resolution after VTE depends on leukocyte migration and angiogenesis, and resembles wound repair. The recruitment of innate immune cells is important in the remodeling process because misguided activation triggers both initiation and propagation of venous thrombosis.29 An impaired resolution process after thrombosis is also associated with PTS and its sequela. Understanding mechanisms regulating thrombus resolution is necessary for the development of effective treatment strategies.
We used Pecam-1−/− mice to investigate the role of this glycoprotein in venous thrombus resolution in a stagnant flow thrombosis model. Our data demonstrate that PECAM-1 deficiency led to significantly larger thrombi at all time points. Mechanisms underlying larger thrombus sizes between days 0 and 3 in Pecam-1−/− mice were not addressed in the present study. Recent findings have emphasized the importance of platelets in venous thrombosis.30 Because platelet PECAM-1 has been shown to inhibit arterial thrombus formation,31 and because PECAM-1 deficient platelets displayed enhanced aggregation and granule secretion in response to various stimuli,32 PECAM-1 deficiency of platelets likely contributed to significantly larger thrombi immediately after ligation.
Similar initial thrombus sizes in controls and Pecam-1−/− mice would have been easier to interpret with regard to resolution rates. However, we calculated volume changes over time. We found that similar size decreases occurred between days 3 to 14 (Figure 1A-D), whereas thrombus volumes increased in Pecam-1−/− mice between days 14 and 28 (Figure 1B). Neutrophil numbers did not differ between controls and Pecam-1−/− thrombi on days 3 and 7 (data not shown), suggesting that similar neutrophil recruitment in the beginning may have contributed to similar resolution rates. However, subsequent thrombus characteristics significantly changed in PECAM-1 deficient thrombi with significantly less macrophages (Figure 1F; Figure 3I-P), less thrombus vessels (Figure 1E and Figure 3A-H) and increased fibrosis (Figure 1H). Endothelial cell PECAM-1 deficiency reduces angiogenesis by inhibition of endothelial cell filopodia.33 Neovascularization and macrophage recruitment are important for thrombus resolution in vivo.34 In our study, PECAM-1 deficiency led to a dampened inflammatory response and less vascularization at the site of thrombosis in FVB/n strain mice, which may account for delayed resolution between days 14 and 28, resulting in thrombus persistence. Histologically, organizing mouse thrombi bear close similarities with nonresolving human thrombi (eg, human complicated DVT and CTEPH).
Based on the murine data of delayed thrombus resolution between days 14 and 28, we aimed to investigate the role of PECAM-1 in a prospective observational clinical study of patients with acute symptomatic DVT. We monitored thrombus resolution via duplex-based thrombus scoring and development of PTS within 12 months. We found significantly elevated baseline sPECAM-1 plasma levels in patients with subsequently delayed thrombus resolution. The difference was also detectable on day 28 (±3), suggesting continued cleavage of PECAM-1, and underscoring the importance of serial blood sampling because the baseline commonly represents a condition in which unspecific inflammatory processes are transiently upregulated.
At first glance the association between elevated baseline sPECAM-1 levels and delayed thrombus resolution seems to contradict the data in the murine model. However, elevated human plasma sPECAM-1 derives exclusively from its cleaved form, which was confirmed by ELISA assays using a monoclonal antibody selectively directed to the PECAM-1 cytoplasmic domain. PECAM-1 must have been cleaved at the surface of cells involved in the resolution process. Previous studies28,35 have demonstrated PECAM-1 proteolytic cleavage at the cell surface and potential competitive inhibition of membrane-bound PECAM-136 during inflammation, thus mimicking a lack of surface expression. This would be in agreement with the observation in mice lacking PECAM-1.
To confirm this hypothesis, we analyzed nonresolving human DVT thrombi in comparison with unthrombosed human venous wall by immunohistochemistry. Domain-specific analysis for the extracellular and intracellular portion of PECAM-1 revealed little co-distribution at the luminal thrombus side, suggesting accumulation of cleaved extracellular PECAM-1, which is most likely the source of elevated sPECAM-1 plasma levels. We suggest that human PECAM-1 activity is suppressed either by its soluble form or by surface shedding. This condition would favor thrombus persistence, similar to the observation in the animal study. Our experiments do not identify the cellular source of sPECAM-1, but we propose that it derives from cells located at the site of thrombosis.
In addition, we evaluated PECAM-1 gene expression in human nonresolving DVT thrombi and chronic thrombi endarterectomized from patients with CTEPH. These thrombi contain more extracellular matrix and less cells compared with the unthrombosed pulmonary artery vessel wall.24 Consequently, housekeeping genes are at a lower level of expression. However, PECAM-1 mRNA levels were normalized to housekeeping genes and still show significant downregulation in nonresolving thrombi, particularly in CTEPH thrombi (Figure 6). It is plausible that decreased numbers of PECAM-1–expressing cells impact expression levels, however, PECAM-1 downregulation on a single cell level cannot be excluded. Based on these data, gene downregulation on an mRNA level is likely to enhance the loss of PECAM-1 activity on a protein level, thus, exacerbating PECAM-1 deficiency.
Persistent thrombus with consecutive vessel occlusion is known to be associated with PTS.37,38 Clinical trials27,39 have focused on soluble cell adhesion molecules (ie, ICAM-1, VCAM-1) in DVT and their potential role as marker molecules for the development of PTS. In our study, univariate regression analysis revealed that baseline sPECAM-1 levels are able to predict delayed thrombus resolution, and also the development of PTS potentially more reliable than sICAM-1. C-reactive protein, an acute-phase protein, did not show a significant difference between the groups (data not shown), indicating that sPECAM-1 results are not simply due to a general inflammatory response.
Patients with Δ thrombus score <4 have a threefold higher risk to develop PTS compared with patients with Δ thrombus score ≥4. If our findings can be confirmed in larger sample size studies, plasma sPECAM-1 level measurement at the time of DVT diagnosis might predict which patients need more aggressive treatment of PTS prevention and which patients possibly benefit from longer periods of anticoagulant treatment, or at least complete compression ultrasonography reevaluation before anticoagulant therapy withdrawal. Based on the novel information in this manuscript, plasma sPECAM-1 level as a biomarker and potential therapeutic implications should be addressed in systematic clinical trials.
This is the first study to provide evidence for the involvement of PECAM-1 in venous thrombus resolution. In a mouse model mimicking human DVT, PECAM-1 deficiency led to significantly larger thrombi and misguided thrombus resolution. In patients with DVT and subsequently delayed thrombus resolution, plasma sPECAM-1 levels are significantly higher, possibly due to enhanced surface cleavage at the site of thrombosis, and predict development of PTS. One limitation of our study is the use of an animal model with a primary deficiency of PECAM-1 comparing with a human model of secondary PECAM-1 deficiency. Our data are further limited by the relatively small sample size and speculation regarding precise mechanisms of sPECAM-1 biological function in patients suffering from nonresolving venous thrombosis. Further studies will have to clarify cellular sources of sPECAM-1 and verify its predictive value.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Sebastian Schellong, University of Dresden, Germany, for his assistance in thrombus scoring, and Dr Peter J. Newman, Blood Research Institute of Wisconsin, Milwaukee for providing monoclonal antibody 235.1.
This work was supported by a fellowship grant of the Austrian Society of Cardiology.
Authorship
Contribution: J.K., B.R., S.A., L.K., M.P.W., and A.P. performed experiments; J.K., I.M.L., and A.W. designed the research; J.K., L.K., A.P., and I.M.L. analyzed the results; J.K. made the tables and figures; J.J. performed complete compression ultrasonography; and J.K. and I.M.L. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests
Correspondence: Irene M. Lang, Department of Cardiology, Medical University of Vienna, Waehringer Guertel 18-20, 1090 Vienna, Austria; e-mail: irene.lang@meduniwien.ac.at.