In this issue of Blood, Nayar et al identify the heat shock protein 90 (Hsp90) oncoproteome and demonstrate efficient antitumor activity in vitro and in vivo upon inhibition of Hsp90 activity in primary effusion lymphoma cells and xenografts, respectively.1
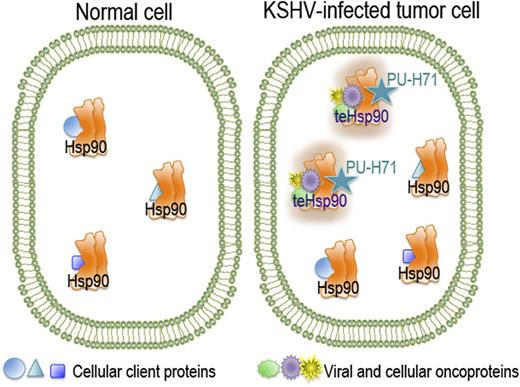
In normal, unstressed cells, the chaperone Hsp90 is bound to its cellular client proteins to promote their correct folding and cell homeostasis. Although Hsp90 is expressed in all cells and tissues, a specific tumor-enriched form of Hsp90 (teHsp90) is found in cancer cells. This oncoprotein-bound form appears as a higher-order multichaperone complex with high affinity for certain Hsp90 inhibitors such as PU-H71.
In normal, unstressed cells, the chaperone Hsp90 is bound to its cellular client proteins to promote their correct folding and cell homeostasis. Although Hsp90 is expressed in all cells and tissues, a specific tumor-enriched form of Hsp90 (teHsp90) is found in cancer cells. This oncoprotein-bound form appears as a higher-order multichaperone complex with high affinity for certain Hsp90 inhibitors such as PU-H71.
Their work brings new hope for fighting this Kaposi sarcoma herpesvirus (KSHV)–induced lymphoma, which is a very aggressive disease with a poor prognosis. It mostly manifests as pleural effusions in Kaposi sarcoma patients with advanced AIDS but can also (rarely) be found in HIV-negative patients. Despite active research and serious efforts to identify the Achilles’ heel of primary effusion lymphoma, it remains a devastating disease for patients and a challenge for clinicians.
Hsp90 is a molecular chaperone that has emerged as an important target in a variety of diseases, including cancer, neurodegenerative diseases, nerve injuries, inflammation, and infection. Hsp90 is often overexpressed in cancer, which makes it an attractive target for novel molecular cancer therapeutic agents. Being the most abundant soluble cytosolic protein even in normal cells in the absence of heat shock (encompassing about 1% of total protein), special attention and precautions are necessary to avoid collateral damage that could result from disruption of the cellular homeostasis in the normal cells. However, this reasonable concern was notably diminished with the discovery of tumor cell–specific subpopulations of Hsp90. By using a small-molecule Hsp90 inhibitor, PU-H71, Moulick et al2 demonstrated that the inhibitor interacts only with a restricted fraction of the chaperone, the tumor-enriched Hsp90 (teHsp90), which is more abundant in cancer cells and, importantly, selective for the Hsp90 that is bound to oncoproteins and co-chaperones (see figure).
The recognition of the clinical potential of targeting the tumor-specific Hsp90 has evoked a number of clinical trials in which different types of Hsp90 inhibitors have been tested on a variety of solid tumors. Despite showing some promising efficacy in phase 2 clinical trials for breast cancer, many of these first-generation inhibitors (geldanamycin and its derivatives 17-AAG [tanespimycin] and 17-DMAG [alvespimycin]) unfortunately induced liver toxicity leading to termination of trials. The promising tumor specificity of the second-generation inhibitors such as PU-H71, a purine-scaffold class derivative, are hoped to reclaim the promise.3 The first-in-human phase 1 trial for treating metastatic solid tumors and lymphomas with PU-H71 was initiated in 2011 (ClinicalTrials.gov Identifier: NCT01393509).
The article by Nayar et al underscores the astonishing capacity of KSHV to pirate vital cellular mechanisms and corroborates previous findings that demonstrate an important role for Hsp90 in controlling expression of viral genes and maintenance of latent infection, both critical processes for the oncogenic virus and cell transformation.4,5 Cells infected with an oncogenic virus are often addicted to both viral and mutated or aberrantly expressed cellular proteins, making them critically reliant on Hsp90. KSHV uses Hsp90 to protect and sustain the activity of viral proteins. However, this clever engagement makes the virus-infected cells vulnerable by sensitizing them to inhibition of the chaperone. Destabilization of viral latent proteins following Hsp90 inhibition provides of powerful and specific way to interfere with the virus-driven deregulation of proliferation and cell intrinsic control mechanisms. In their article, Nayar et al combined Hsp90 inhibition with targeting of antiapoptotic Bcl-2, one of the key players in pathways identified in the Hsp90 oncoproteome, which further augmented the antitumor potency of the Hsp90 inhibitor. Other cancer critical pathways discovered in the proteomic approach, such as nuclear factor κB and phosphatidylinositol 3-kinase/mammalian target of rapamycin, represent additional targets to be tested in combination with PU-H71. Other oncogenic viruses are exploiting Hsp90-mediated chaperoning to control virus-induced signal transduction and virus–host protein interactions.6 It is therefore tempting to speculate that Hsp90 inhibition would also represent an attractive novel therapeutic strategy for other viral cancers.
The study by Nayar et al highlights the now widely recognized, strong potential of combinatorial therapeutic strategies in combating cancer. Because tumor cells often develop resistance to even the most potent single targeted therapy, the chances for successfully eradicating the tumor are significantly higher when the treatment modality is directed against two pathways critical for cancer cell survival.
Conflict-of-interest disclosure: The author declares no competing financial interests.