Key Points
Life span estimates can be sensitive to the duration of stable isotope label administration, explaining discrepancies in the literature.
Multiexponential models are needed to obtain reliable leukocyte life span estimates.
Abstract
Quantitative knowledge of the turnover of different leukocyte populations is a key to our understanding of immune function in health and disease. Much progress has been made thanks to the introduction of stable isotope labeling, the state-of-the-art technique for in vivo quantification of cellular life spans. Yet, even leukocyte life span estimates on the basis of stable isotope labeling can vary up to 10-fold among laboratories. We investigated whether these differences could be the result of variances in the length of the labeling period among studies. To this end, we performed deuterated water-labeling experiments in mice, in which only the length of label administration was varied. The resulting life span estimates were indeed dependent on the length of the labeling period when the data were analyzed using a commonly used single-exponential model. We show that multiexponential models provide the necessary tool to obtain life span estimates that are independent of the length of the labeling period. Use of a multiexponential model enabled us to reduce the gap between human T-cell life span estimates from 2 previously published labeling studies. This provides an important step toward unambiguous understanding of leukocyte turnover in health and disease.
Introduction
Quantitative insights into leukocyte turnover is vital to a better understanding of immune function in health and disease.1,2 These insights help understand the pathogenesis and treatment of clinical conditions of leukocyte depletion, such as HIV infection,3 bone marrow transplantation, or chemotherapy; and leukocyte excess, including leukemia.4 In vivo stable isotope labeling with deuterated (heavy) water (2H2O) or deuterated glucose is one of the most reliable ways to measure leukocyte turnover, because deuterium labeling can be safely applied in humans and does not interfere with cell turnover at the doses used.2,5 Nevertheless, T-cell life span estimates can differ up to 10-fold among stable isotope-labeling studies.2 The cause of this discrepancy has yet to be elucidated.
A meta-analysis of different stable isotope-labeling studies revealed a positive correlation between the estimated T-cell life span and the duration of label administration.2 Studies based on deuterated glucose, which is typically administered for shorter periods than 2H2O, have consistently yielded shorter average life spans than studies based on heavy water.2 Although it cannot be excluded that a difference between the 2H-labeled compounds may have an influence,1 the correlation between the expected life span and the length of the labeling period remained when comparing life span estimates derived from glucose labeling or water labeling separately.2 This finding suggests that at least some of the discrepancy between estimated T-cell life spans from stable isotope-labeling studies is attributable to the duration of label administration.
Mathematical modeling is essential for the interpretation of stable isotope-labeling data. Typically, these models are differential equations, which are based on the assumption that cellular events such as division and death are distributed exponentially. Depending on the complexity of the population structure of the model, its solution involves 1 to several exponentials. Therefore, we refer to these models as exponential models. A major step forward was made by Asquith et al,6 who argued that if a cell population is kinetically heterogeneous (ie, a population comprising multiple subpopulations with different turnover rates, or a population in which recently divided cells and quiescent cells have different life expectancies), the rate of label uptake during the labeling period may not be equal to the rate at which label is lost after label cessation, because the kinetics of labeled cells may not be representative of the cell population as a whole. To account for this heterogeneity, a model was proposed that distinguishes 2 parameters: the average turnover rate (p), and the average disappearance rate of labeled cells (d*). This model is now commonly used4,7-11 and has stressed the importance of deducing average life spans from p. The rate of d* has previously been shown to be dependent on the length of the labeling period: the shorter the labeling period, the stronger the bias toward rapidly proliferating cells in the labeled fraction, and hence the faster the loss of label d*.6 To date, why also p is higher (and hence the average life span shorter) in short-term compared with longer-term labeling studies2 has not been experimentally addressed.
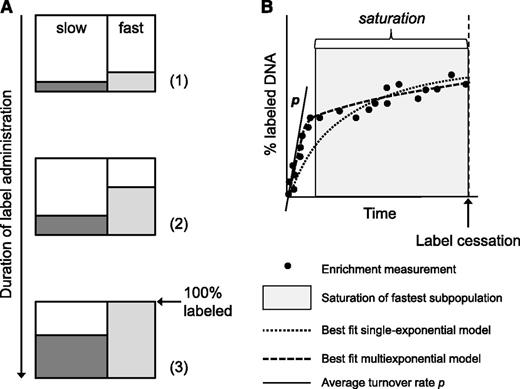
Unlike delabeling curves, which may vary according to the length of the labeling period, the shape of the uplabeling curve is independent of the length of the labeling period. It is determined by the weighted average of turnover rates of all subpopulations, and its initial slope should reflect p (Figure 1A). Here, we test the hypothesis that during long-term labeling, the label uptake of cells with fast turnover may start to saturate7 (Figure 1A). If label administration is continued beyond this point, subsequent label accrual occurs mainly because of cells with relatively slow kinetics. Because single-exponential models cannot capture the saturation behavior and, instead, are forced to make a compromise, p could become increasingly underestimated as the length of the labeling period increases (Figure 1B). This mechanism might explain the positive correlation between the estimated T-cell life span and the length of the labeling period observed in the literature.
The influence of the length of the labeling period on the estimated turnover rate p. Consider an artificial long-term labeling experiment of a kinetically heterogeneous cell population, in which the labeled fractions of a slow (dark gray) and a fast subpopulation (light gray) gradually increase with the duration of label administration (A). During labeling, samples are obtained, and the percentage of labeled DNA is determined at several time points (B, black circles). During the labeling phase, the initial accrual of label (the slope nearby the origin, as indicated by the black tangent line) reflects p of the kinetically heterogeneous population (A, situations 1 and 2; B, white area). If labeling is continued, the enrichment level of the fastest subpopulation may start to saturate (A, situation 3). Although cells of the fastest subpopulation continue to divide after this point, this is no longer reflected by a corresponding increase in their enrichment level. If sampling is continued (B, gray area), any further increase in labeled DNA is largely the result of cell production in the slow subpopulation, reflected by a second, flatter slope of the labeling curve (B). If the label enrichment data are fitted using a single-exponential model (dotted black curve), the model seeks a compromise between the initial, steep increase and the later, slower increase of label enrichment. As a result, the model fit is forced to bend downward from the initial slope, and the average turnover rate will become increasingly underestimated with increasing duration of label administration. In contrast, the multiexponential model corrects for this effect by allowing for multiple slopes during the labeling phase (B, dashed black curve), and thereby yields a reliable estimate of the average turnover rate, independent of the length of the labeling period.
The influence of the length of the labeling period on the estimated turnover rate p. Consider an artificial long-term labeling experiment of a kinetically heterogeneous cell population, in which the labeled fractions of a slow (dark gray) and a fast subpopulation (light gray) gradually increase with the duration of label administration (A). During labeling, samples are obtained, and the percentage of labeled DNA is determined at several time points (B, black circles). During the labeling phase, the initial accrual of label (the slope nearby the origin, as indicated by the black tangent line) reflects p of the kinetically heterogeneous population (A, situations 1 and 2; B, white area). If labeling is continued, the enrichment level of the fastest subpopulation may start to saturate (A, situation 3). Although cells of the fastest subpopulation continue to divide after this point, this is no longer reflected by a corresponding increase in their enrichment level. If sampling is continued (B, gray area), any further increase in labeled DNA is largely the result of cell production in the slow subpopulation, reflected by a second, flatter slope of the labeling curve (B). If the label enrichment data are fitted using a single-exponential model (dotted black curve), the model seeks a compromise between the initial, steep increase and the later, slower increase of label enrichment. As a result, the model fit is forced to bend downward from the initial slope, and the average turnover rate will become increasingly underestimated with increasing duration of label administration. In contrast, the multiexponential model corrects for this effect by allowing for multiple slopes during the labeling phase (B, dashed black curve), and thereby yields a reliable estimate of the average turnover rate, independent of the length of the labeling period.
Here, we have investigated this hypothesis by performing 2H2O labeling experiments in mice in which only the duration of label administration was varied. We confirm that life spans estimated by fitting single-exponential models to stable isotope labeling data are sensitive to the length of the labeling period. When using a multiexponential model (describing label accrual with more than 1 exponential), which explicitly captures kinetic heterogeneity within a cell population12 (Figure 1B), we found that life span estimates became independent of the duration of label administration. By labeling mice in utero to have all leukocytes of newborn mice equally labeled, we confirmed that the life span estimates that were obtained with the multiexponential model were reliable. Application of the model to published human deuterium-labeling data reduced the difference between life span estimates on the basis of glucose and 2H2O labeling studies.10,13 Both our findings and approach present a major step toward consensus on how long different types of leukocytes live in health and disease.
Methods
Mice
C57Bl/6 mice were maintained by in-house breeding at the Central Animal Facility at Utrecht University, Utrecht, The Netherlands, in accordance with institutional and national guidelines.
2H2O labeling
For finite-term labeling experiments, ∼12-week-old male mice were given a boost intraperitoneal injection of 15 mL/kg of 2H2O (99.8%; Cambridge Isotopes) in phosphate-buffered saline and received 4% 2H2O for 1, 4, or 8 weeks. For prenatal labeling experiments, to obtain mice in which all cells were labeled to the same extent, female mice were given a boost injection of 15 mL/kg of 99.8% 2H2O in phosphate-buffered saline. They were placed together with male mice and drank 4% 2H2O until they gave birth. After weaning, male pups received 4% 2H2O until age 16 weeks.
The 9-week labeling data in humans have been published previously,10 and the enrichment in total CD4+ T cells was derived from naive and memory T cells as described in supplemental Materials (available on the Blood Web site).
Cell preparation and sorting
Spleens were isolated at different time points for further sorting. Thymocytes were isolated as a cell population with rapid turnover to determine the maximal level of label enrichment. Blood was collected in EDTA vials and was spun down to isolate plasma. Single-cell suspensions were obtained as described previously.10 Splenocytes were stained with CD62L-FITC, CD44-eFluor450 (eBioScience, San Diego, CA), CD4-APC-H7, and CD8-PerCP (BD PharMingen, San Jose, CA) in the presence of a 2.4G2-blocking antibody. Within CD4+CD8− and CD4−CD8+ splenocytes, naive T cells were defined as CD62L+CD44− and effector/memory T cells as CD44+. Cells were sorted using a FACSAria cell sorter and FACSdiva software (BD PharMingen). Genomic DNA was isolated according to the manufacturer’s instructions (Nucleospin Blood QuickPure; Macherey-Nagel, Duren, Germany).
Measurement of deuterium enrichment in DNA and body water
Deuterium enrichment in DNA was measured according to the method described by Neese et al14 with minor modifications, as described previously.10 Both natural enrichment and concentration dependence (abundance sensitivity) were controlled for, using a naturally enriched background sample or standards of known isotopic enrichments. To determine deuterium enrichment in body water, plasma samples were measured using gas chromatography-mass spectrometry.15,16 Mathematical modeling is described in supplemental Materials.
Statistical analysis
Analysis-of-variance tests were performed to compare the different estimates using Prism 5 (GraphPad). Nested mathematical models were compared using an F test. Differences with a P value of < .05 were considered significant.
Results
Life span estimates can be influenced by the duration of label administration
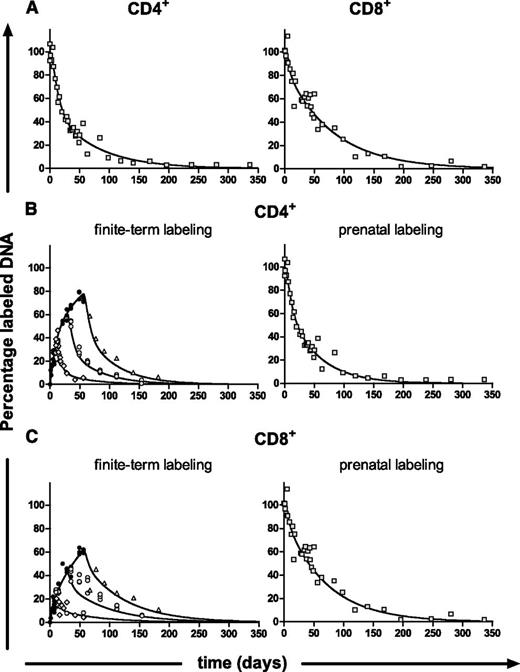
The observed correlation in the literature between average life span estimates and the duration of label administration2 prompted us to investigate whether we could reproduce this correlation within a single experiment, in which only the duration of label administration was varied. Twelve-week-old C57Bl/6 mice were given a bolus of 2H2O and subsequently 4% 2H2O in the drinking water for 1, 4, or 8 weeks. Splenic effector/memory (CD44+) T cells and naive (CD62L+CD44-) T cells were isolated at different time points during 2H2O administration (labeling phase), and after 2H2O administration (delabeling phase), and deuterium enrichment in the DNA was measured. Labeling curves for naive (Figure 2A) and effector/memory (Figure 2B) CD4+ and CD8+ T-cell subsets of the 1-week, 4-week, and 8-week labeling experiments were fitted separately with the single-exponential model proposed for interpreting deuterated glucose experiments,6 which we have previously adapted for use with 2H2O labeling10 (supplemental Materials).
Best fits of the single-exponential model and the multiexponential model to labeling experiments of different duration. At different time points during and after labeling, the percentage of labeled DNA of splenic (A) naive and (B-C) effector/memory (E/M) CD4+and CD8+ T cells was determined. Dots represent measurements (ie, individual mice) at different time points during labeling for 1, 4, and 8 weeks (●, black circles), and during delabeling after 1 week of labeling ( , gray diamonds), 4 weeks of labeling (
, gray diamonds), 4 weeks of labeling ( , gray circles), or 8 weeks of labeling (
, gray circles), or 8 weeks of labeling ( , gray triangles). (A-B) Data were fitted separately for each labeling period using the single-exponential (SE) model to estimate p of the cells and d* of the labeled cells for the corresponding labeling period. For naive T cells, a delay was added in the model as described in supplemental Materials equations 9 and 10 and was estimated to be 4 days (95% CI, 2-6 days). For effector/memory T cells (B), the best-fitting curves during the labeling period were not identical for the different labeling periods (indicated by arrows). (C) When the data were fitted separately for each labeling period using the multiexponential (ME) model (describing 2 kinetically different subpopulations; the addition of more subpopulations did not change the average turnover rate), the best-fitting curves during the labeling period were almost identical. Label enrichment was corrected for 2H2O enrichment in plasma (supplemental Figure 1A) and was scaled between 0% and 100% by normalizing for the maximal percentage of labeled DNA as measured in thymocytes (supplemental Figure 1B).
, gray triangles). (A-B) Data were fitted separately for each labeling period using the single-exponential (SE) model to estimate p of the cells and d* of the labeled cells for the corresponding labeling period. For naive T cells, a delay was added in the model as described in supplemental Materials equations 9 and 10 and was estimated to be 4 days (95% CI, 2-6 days). For effector/memory T cells (B), the best-fitting curves during the labeling period were not identical for the different labeling periods (indicated by arrows). (C) When the data were fitted separately for each labeling period using the multiexponential (ME) model (describing 2 kinetically different subpopulations; the addition of more subpopulations did not change the average turnover rate), the best-fitting curves during the labeling period were almost identical. Label enrichment was corrected for 2H2O enrichment in plasma (supplemental Figure 1A) and was scaled between 0% and 100% by normalizing for the maximal percentage of labeled DNA as measured in thymocytes (supplemental Figure 1B).
Best fits of the single-exponential model and the multiexponential model to labeling experiments of different duration. At different time points during and after labeling, the percentage of labeled DNA of splenic (A) naive and (B-C) effector/memory (E/M) CD4+and CD8+ T cells was determined. Dots represent measurements (ie, individual mice) at different time points during labeling for 1, 4, and 8 weeks (●, black circles), and during delabeling after 1 week of labeling ( , gray diamonds), 4 weeks of labeling (
, gray diamonds), 4 weeks of labeling ( , gray circles), or 8 weeks of labeling (
, gray circles), or 8 weeks of labeling ( , gray triangles). (A-B) Data were fitted separately for each labeling period using the single-exponential (SE) model to estimate p of the cells and d* of the labeled cells for the corresponding labeling period. For naive T cells, a delay was added in the model as described in supplemental Materials equations 9 and 10 and was estimated to be 4 days (95% CI, 2-6 days). For effector/memory T cells (B), the best-fitting curves during the labeling period were not identical for the different labeling periods (indicated by arrows). (C) When the data were fitted separately for each labeling period using the multiexponential (ME) model (describing 2 kinetically different subpopulations; the addition of more subpopulations did not change the average turnover rate), the best-fitting curves during the labeling period were almost identical. Label enrichment was corrected for 2H2O enrichment in plasma (supplemental Figure 1A) and was scaled between 0% and 100% by normalizing for the maximal percentage of labeled DNA as measured in thymocytes (supplemental Figure 1B).
, gray triangles). (A-B) Data were fitted separately for each labeling period using the single-exponential (SE) model to estimate p of the cells and d* of the labeled cells for the corresponding labeling period. For naive T cells, a delay was added in the model as described in supplemental Materials equations 9 and 10 and was estimated to be 4 days (95% CI, 2-6 days). For effector/memory T cells (B), the best-fitting curves during the labeling period were not identical for the different labeling periods (indicated by arrows). (C) When the data were fitted separately for each labeling period using the multiexponential (ME) model (describing 2 kinetically different subpopulations; the addition of more subpopulations did not change the average turnover rate), the best-fitting curves during the labeling period were almost identical. Label enrichment was corrected for 2H2O enrichment in plasma (supplemental Figure 1A) and was scaled between 0% and 100% by normalizing for the maximal percentage of labeled DNA as measured in thymocytes (supplemental Figure 1B).
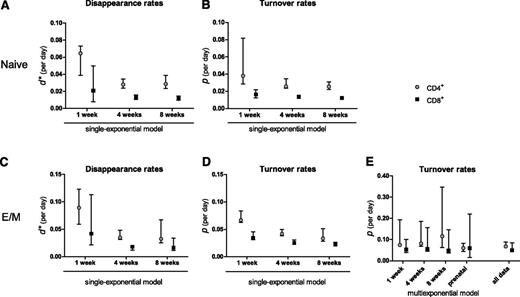
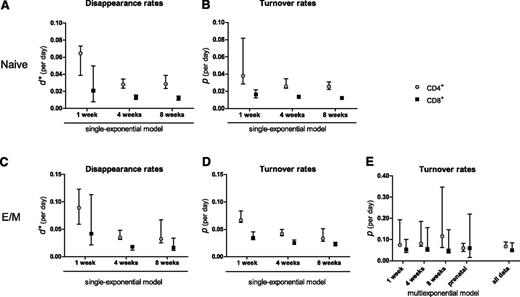
For naive CD4+ and CD8+ T cells, d* (Figure 3A) was somewhat higher than the estimated p (Figure 3B), especially in the 1-week labeling experiment, and tended to decrease with the length of the labeling period, although not significantly (P = .07 for CD4+ and P = .19 for CD8+; Figure 3A). The estimated p (and hence the average life span) of naive T cells was similar for the 3 durations of label administration (P = .30 for CD4+ and P = .41 for CD8+; Figure 3B). For CD4+ and CD8+ effector/memory T cells, d* decreased as the length of the labeling period increased (P < 10−4; Figure 3C), and unlike what was observed for naive T cells, even the estimated average turnover rate p decreased significantly as the duration of label administration increased (P < 10−4; Figure 3D). Hence, within a single experiment, we reproduced the previously observed correlation in the literature between the duration of label administration and the estimated average life span.2
Summary of parameter estimates. Estimates of d* (A,C) and the average turnover rate p (B,D) of naive (A-B) and effector/memory (C-D) CD4+ ( , gray circles) and CD8+ (▪, black squares) T cells, obtained by fitting the single-exponential model to the data collected during 1, 4, or 8 weeks of labeling. (E) Estimates of the average turnover rate of CD4+ (
, gray circles) and CD8+ (▪, black squares) T cells, obtained by fitting the single-exponential model to the data collected during 1, 4, or 8 weeks of labeling. (E) Estimates of the average turnover rate of CD4+ ( , gray circles) and CD8+ (▪, black squares) effector/memory T cells obtained by fitting a multiexponential model (describing 2 subpopulations) to the individual data sets (labeling for 1, 4, or 8 weeks; or prenatal labeling) and simultaneously to all data combined. Bars with whiskers represent the 95% CIs of the estimates obtained by bootstrapping the residuals.
, gray circles) and CD8+ (▪, black squares) effector/memory T cells obtained by fitting a multiexponential model (describing 2 subpopulations) to the individual data sets (labeling for 1, 4, or 8 weeks; or prenatal labeling) and simultaneously to all data combined. Bars with whiskers represent the 95% CIs of the estimates obtained by bootstrapping the residuals.
Summary of parameter estimates. Estimates of d* (A,C) and the average turnover rate p (B,D) of naive (A-B) and effector/memory (C-D) CD4+ ( , gray circles) and CD8+ (▪, black squares) T cells, obtained by fitting the single-exponential model to the data collected during 1, 4, or 8 weeks of labeling. (E) Estimates of the average turnover rate of CD4+ (
, gray circles) and CD8+ (▪, black squares) T cells, obtained by fitting the single-exponential model to the data collected during 1, 4, or 8 weeks of labeling. (E) Estimates of the average turnover rate of CD4+ ( , gray circles) and CD8+ (▪, black squares) effector/memory T cells obtained by fitting a multiexponential model (describing 2 subpopulations) to the individual data sets (labeling for 1, 4, or 8 weeks; or prenatal labeling) and simultaneously to all data combined. Bars with whiskers represent the 95% CIs of the estimates obtained by bootstrapping the residuals.
, gray circles) and CD8+ (▪, black squares) effector/memory T cells obtained by fitting a multiexponential model (describing 2 subpopulations) to the individual data sets (labeling for 1, 4, or 8 weeks; or prenatal labeling) and simultaneously to all data combined. Bars with whiskers represent the 95% CIs of the estimates obtained by bootstrapping the residuals.
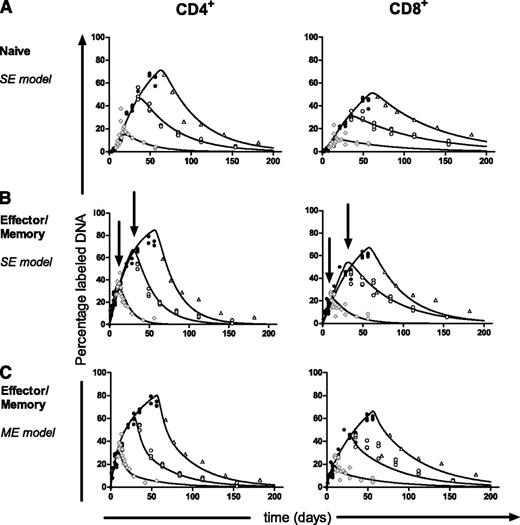
Inspection of the best fits to the data shows that the discrepancy in the estimated average turnover rate of effector/memory T cells is caused by the model that was fitted to the data. Although the curves of the 3 labeling experiments should be identical during labeling (as we observed for naive T cells, Figure 2A), the separate fits to the 1-, 4-, and 8-week labeling data of effector/memory T cells differed during labeling (Figure 2B, indicated by arrows). Apparently, the model could not capture saturation of the fastest cells in the effector/memory pool and thereby underestimated the average turnover rate during long-term labeling (Figure 1).
Multiexponential models correct for the influence of the length of the labeling period
Because the correlation between p and the duration of label administration was most evident for the CD4+ and CD8+ effector/memory T-cell pools, we used the labeling data from those cell populations to investigate how life spans can be determined reliably when cell populations are kinetically heterogeneous. We propose using a multiexponential model that explicitly accounts for kinetic heterogeneity,12 by describing multiple subpopulations each with their own production and disappearance rate. Each subpopulation is assumed to be in equilibrium, that is, its production equals loss (supplemental Materials). In contrast to the single-exponential models that are typically used, a multiexponential model describes both the labeling and the delabeling phase by a multiexponential function.12 Because a very similar model is obtained in populations with temporal heterogeneity (eg, consisting of quiescent and activated cells),17 we here use the multiexponential model generally to account for heterogeneity in the population. Although the number of kinetically different subpopulations within a cell population may not be known, one can increase the number of subpopulations in the model until the estimated average turnover rate no longer markedly changes, provided sufficient data are available (supplemental Materials).
To test whether a multiexponential model would correct for the influence of the length of label administration on the estimated average turnover rate, we fitted the individual labeling curves of T cells from the 1-, 4-, and 8-week labeling experiments separately using a multiexponential model. Because the multiexponential model did not improve the fits of the naive T-cell data for any duration of label administration (not shown) but did influence the average life span estimates of effector/memory T cells, we decided to focus on the latter. The labeling data from the CD4+ and CD8+effector/memory T-cell pools were well described by a model describing 2 kinetically different subpopulations (the addition of more subpopulations did not change the average turnover rate). In contrast to the single-exponential model (Figure 2B), the multiexponential model described the 1-, 4-, and 8-week labeling data with largely overlapping curves during labeling (Figure 2C) and thus yielded 3 similar turnover rates (P = .08 for CD4+ and P = .60 for CD8+) that were independent of the length of the labeling period (Figure 3E). As expected, the estimates obtained by the single- and multiexponential model differed most for the longer labeling periods, and the multiexponential model gave significantly better fits to the data than the single-exponential model (8 weeks of labeling: P < .0001 for CD4+ and P = .0028 for CD8+; 4 weeks of labeling: P < .0001 for CD4+). For the 1-week data, both models behaved similarly.
It should be noted that the 4-week labeling data of CD8+ effector/memory T cells were not fitted well during the delabeling phase, and that the multiexponential model did not describe the 4-week labeling data significantly better than the single-exponential model (P = .08). Importantly, our estimates of the average turnover rate of CD8+ effector/memory T cells do not depend on the 4-week labeling data, as using only 1-week and 8-week labeling data (separately or combined) yielded consistent estimates.
Hence, the correlation of published turnover rates with the length of the labeling period may be the result of kinetic heterogeneity that was not fully accounted for by the models that were used to fit the data. Mathematical models that explicitly capture such kinetic heterogeneity yield average turnover rates that do not depend on the duration of label administration.
Prenatal labeling experiments yield similar life span estimates
Next, we sought to obtain independent confirmation of the turnover rates that we estimated when fitting the multiexponential model to the 1-, 4-, and 8-week labeling data. Because the main difficulty in the interpretation of “finite-term” labeling experiments is caused by the difference between cells that are and are not labeled during the experiment, we designed a labeling experiment in which, at cessation of label administration, all cells were labeled. Female mice received a bolus of 2H2O and were subsequently fed with 4% 2H2O in the drinking water before conception and throughout pregnancy. Female mice thus gave birth to pups that had been labeled in utero (referred to as “prenatal labeling”) and in which all cells were equally labeled. Pups received 2H2O until age 16 weeks, after which 2H2O was withdrawn from the drinking water. They were euthanized at different time points after labeling to measure the loss of deuterium enrichment in the DNA of their T cells. The resulting delabeling curves were used to deduce d, which, in this case, reflects the cell population as a whole and can directly be interpreted as the average turnover rate.
In line with the finite-term labeling experiments, fitting the multiexponential model (describing 2 kinetically different subpopulations; the addition of more subpopulations did not change the average turnover rate, Figure 4A) to the effector/memory delabeling data gave a significantly better description of the data (P < .01) than the single-exponential model (not shown). Fitting the prenatal data yielded average turnover rates for effector/memory CD4+ and CD8+ T cells that were similar to the estimates obtained in the 1-, 4-, and 8-week labeling experiments (P = .68 for CD4+ and P = .54 for CD8+; Figure 3E). Indeed, we found that delabeling of a fully labeled population behaved similarly to labeling of an unlabeled population. For all cell subsets analyzed, both the average turnover rate and the number of exponentials required to describe the data were similar for the finite-term labeling data and the corresponding prenatal labeling data. This independent experiment confirmed the turnover estimates obtained by fitting the multiexponential model to the finite-term labeling data above.
Best fits of the multiexponential model to effector/memory T-cell data from prenatal and finite-term labeling experiments. (A) Mice were labeled prenatally and drank 2H2O until age 16 weeks. At different time points after label cessation, the percentage of labeled DNA of splenic effector/memory CD4+ (left) and CD8+ T cells (right) was determined. Gray squares ( ) represent measurements (ie, individual mice) at different time points after labeling. Data were fitted with the multiexponential model (describing 2 subpopulations) to estimate the average turnover rate of the total cell population. (B-C) Effector/memory CD4+ (B) and CD8+ (C) T-cell labeling data from the finite-term (left) and prenatal labeling (right) experiments were simultaneously fitted with the multiexponential model to estimate the average turnover rate. Black circles (●) represent measurements (ie, individual mice) at different time points during labeling; during delabeling after 1 week of labeling (
) represent measurements (ie, individual mice) at different time points after labeling. Data were fitted with the multiexponential model (describing 2 subpopulations) to estimate the average turnover rate of the total cell population. (B-C) Effector/memory CD4+ (B) and CD8+ (C) T-cell labeling data from the finite-term (left) and prenatal labeling (right) experiments were simultaneously fitted with the multiexponential model to estimate the average turnover rate. Black circles (●) represent measurements (ie, individual mice) at different time points during labeling; during delabeling after 1 week of labeling ( , gray diamonds), 4 weeks of labeling (
, gray diamonds), 4 weeks of labeling ( , gray circles), or 8 weeks of labeling (
, gray circles), or 8 weeks of labeling ( , gray triangles); and during delabeling of prenatally labeled mice (
, gray triangles); and during delabeling of prenatally labeled mice ( , gray squares).
, gray squares).
Best fits of the multiexponential model to effector/memory T-cell data from prenatal and finite-term labeling experiments. (A) Mice were labeled prenatally and drank 2H2O until age 16 weeks. At different time points after label cessation, the percentage of labeled DNA of splenic effector/memory CD4+ (left) and CD8+ T cells (right) was determined. Gray squares ( ) represent measurements (ie, individual mice) at different time points after labeling. Data were fitted with the multiexponential model (describing 2 subpopulations) to estimate the average turnover rate of the total cell population. (B-C) Effector/memory CD4+ (B) and CD8+ (C) T-cell labeling data from the finite-term (left) and prenatal labeling (right) experiments were simultaneously fitted with the multiexponential model to estimate the average turnover rate. Black circles (●) represent measurements (ie, individual mice) at different time points during labeling; during delabeling after 1 week of labeling (
) represent measurements (ie, individual mice) at different time points after labeling. Data were fitted with the multiexponential model (describing 2 subpopulations) to estimate the average turnover rate of the total cell population. (B-C) Effector/memory CD4+ (B) and CD8+ (C) T-cell labeling data from the finite-term (left) and prenatal labeling (right) experiments were simultaneously fitted with the multiexponential model to estimate the average turnover rate. Black circles (●) represent measurements (ie, individual mice) at different time points during labeling; during delabeling after 1 week of labeling ( , gray diamonds), 4 weeks of labeling (
, gray diamonds), 4 weeks of labeling ( , gray circles), or 8 weeks of labeling (
, gray circles), or 8 weeks of labeling ( , gray triangles); and during delabeling of prenatally labeled mice (
, gray triangles); and during delabeling of prenatally labeled mice ( , gray squares).
, gray squares).
Simultaneously fitting the model to the complete data set we had collected (ie, the 1-, 4-, and 8-week labeling experiments and the prenatal labeling experiments together, Figure 4B-C) revealed that mouse CD4+ and CD8+ effector/memory T cells have average turnover rates of 0.068 (95% confidence interval [CI], 0.065-0.088) and 0.050 (95% CI, 0.045-0.085) per day (Figure 3E), corresponding to average life spans of 15 days (95% CI, 11-15 days) for CD4+, and 20 days (95% CI, 12-22 days) for CD8+ effector/memory T cells (Table 1).
The multiexponential model reduces the difference between human T-cell life span estimates
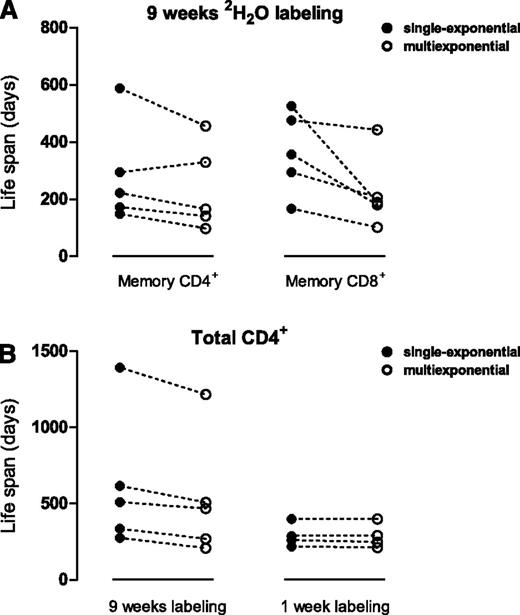
Next, we applied the multiexponential model to previously published human 2H2O labeling data10 to obtain human T-cell life span estimates that are independent of the length of the labeling period. For each individual labeled with 2H2O for 9 weeks, the multiexponential model (describing 2 kinetically different subpopulations; the addition of more subpopulations did not change the average turnover rate) fitted the memory CD4+ and CD8+ T-cell data significantly better (P < .001) and, in general, yielded higher average turnover rates p (and therefore shorter average life spans) than the single-exponential model (Figure 5A; supplemental Figure 4). Hence, single-exponential models have overestimated the average life span of memory T cells in long-term labeling studies. According to the multiexponential model, human memory CD4+ and CD8+ T cells have a median p of 0.0061 (range, 0.0020-0.0141) and 0.0064 (range, 0.0043-0.089) per day, corresponding to median life spans of 164 days (range,71-500 days) for CD4+ and 157 days (range, 113-231 days) for CD8+ memory T cells.
Average T-cell life spans re-estimated from published labeling experiments in humans. Data from 2 published experiments were used: 9-week 2H2O labeling in 5 healthy individuals,10 and 1-week deuterated glucose labeling in 4 healthy individuals.13 For each individual, the life span of the indicated cell population was estimated using a single-exponential model (●, closed circles) or a multiexponential model (○, open circles, describing 2 kinetically different subpopulations; the addition of more subpopulations did not change the average turnover rate). Estimates obtained for a single individual using the 2 models are connected by a dashed line (‐‐‐). (A-B) For each individual in the 9-week 2H2O-labeling experiment, the average life span of CD4+ and CD8+ memory T cells was estimated using both models. Individual fits of the multiexponential model to the memory T-cell data are shown in supplemental Figure 4. (B) The average life span of total CD4+ T cells was re-estimated by fitting the single-exponential model (●, closed circles) or the multiexponential model (○, open circles) to the deuterium-labeling data of individuals labeled with deuterated glucose for 1 week,13 or 2H2O for 9 weeks.10 For the 9-week labeling experiment, individual fits of the multiexponential model are shown in supplemental Figure 5. For the 1-week glucose-labeling data, both models included a 1-day delay.17,22
Average T-cell life spans re-estimated from published labeling experiments in humans. Data from 2 published experiments were used: 9-week 2H2O labeling in 5 healthy individuals,10 and 1-week deuterated glucose labeling in 4 healthy individuals.13 For each individual, the life span of the indicated cell population was estimated using a single-exponential model (●, closed circles) or a multiexponential model (○, open circles, describing 2 kinetically different subpopulations; the addition of more subpopulations did not change the average turnover rate). Estimates obtained for a single individual using the 2 models are connected by a dashed line (‐‐‐). (A-B) For each individual in the 9-week 2H2O-labeling experiment, the average life span of CD4+ and CD8+ memory T cells was estimated using both models. Individual fits of the multiexponential model to the memory T-cell data are shown in supplemental Figure 4. (B) The average life span of total CD4+ T cells was re-estimated by fitting the single-exponential model (●, closed circles) or the multiexponential model (○, open circles) to the deuterium-labeling data of individuals labeled with deuterated glucose for 1 week,13 or 2H2O for 9 weeks.10 For the 9-week labeling experiment, individual fits of the multiexponential model are shown in supplemental Figure 5. For the 1-week glucose-labeling data, both models included a 1-day delay.17,22
Finally, we investigated whether the multiexponential model could resolve the discrepancy between previously published human stable isotope-labeling studies. We confined our analysis to 2 data sets that clearly differed in the length of the labeling period, and had a sufficient number of data points: a 1-week deuterated glucose-labeling experiment13 and a 9-week 2H2O-labeling experiment.10 Because the glucose-labeling experiment reported deuterium enrichment in total CD4+ T cells, and the 2H2O-labeling experiment distinguished between naive and memory T cells, we first recalculated the corresponding levels of deuterium enrichment in total CD4+ T cells for the 9-week 2H2O-labeling experiment (supplemental Materials). When we fitted a single-exponential model to the data, the life spans estimated from the 9-week labeling experiment were longer than the life spans based on the 1-week labeling experiment (Figure 5B; individual fits in supplemental Figure 5), in line with the positive correlation between estimated life spans and the duration of label administration in the literature.2 The multiexponential model reduced the estimated life spans from the 9-week labeling experiment and hence reduced the differences between the studies (Figure 5B). Although the discrepancies between the 2 studies were not entirely resolved, largely because of a single outlier in the 2H2O study whose memory turnover was much lower than in the other individuals10 (supplemental Figure 4D), correction of the length-of-labeling effect revealed that the current best estimate of the average life span of CD4+ T cells in healthy human adults (based on the median values of both studies) lies between 270 and 469 days.
Discussion
The quantification of leukocyte life spans from stable isotope-labeling data relies on the use of mathematical models. The finite-term labeling experiments that we performed in mice show that single-exponential models fail to correctly describe the dynamics of kinetically heterogeneous cell populations and thereby yield average life span estimates that depend on the duration of label administration. Longer labeling periods gave rise to longer estimated average life spans, confirming the correlation observed in the literature.2 These analyses suggest that a considerable part of the discrepancy in published T-cell life spans arises from differences in the duration of label administration. Here, we show that the use of a multiexponential model resolves the dependence on the length of the labeling period and thereby yields reliable turnover parameters.
It has been proposed before that a multiexponential model describing all subpopulations would be the ideal way to model kinetically heterogeneous cell populations, but that this would lead to models with too many parameters.6 The mathematical model proposed by Asquith et al6 was a pragmatic solution that captured kinetic heterogeneity by allowing p to be different from d*. However, the fact that single-exponential models fail to describe labeling curves that are identical during labeling but different after label cessation stresses the need for a multiexponential model to obtain reliable estimates of turnover rates, particularly when the labeling period is long.
Fitting multiexponential models not only reveals the average life span of a cell population but also reveals quantitative insights into the sizes αi and turnover rates pi of its subpopulations. The uncertainty on the latter parameters is, however, generally much larger than on p, because of the strong correlation between the size of a subpopulation and its turnover rate (supplemental Figure 6). Therefore, the biological interpretation of the parameters describing the kinetically different subpopulations used in the model is not straightforward. It is important to realize that the number of kinetically different subpopulations that is sufficient to describe the data may, in fact, be lower than the actual number of subpopulations, and that the subpopulations need not even reflect phenotypically different subsets. Moreover, if the use of a multiexponential model significantly improves the fit to the data, an alternative interpretation is that cells transiently have different turnover rates (ie, that there is so-called temporal heterogeneity). For example, resting cells and cells that have recently been produced or activated may have different life expectancies, an illustrative example being activation-induced cell death.7,17
Our analyses demonstrate that the use of single-exponential models may lead to overestimation of the average life span of kinetically heterogeneous cell populations, especially in long-term labeling studies. This problem can be overcome by implementing a multiexponential model. Hence, both short-term and long-term labeling can be considered, and the decision should be based on the population of interest (slow or fast turnover) and on practical and/or ethical considerations. Although short-term labeling studies are less prone to underestimate cellular turnover rates, long-term labeling can also have advantages. First, longer labeling periods allow more frequent sampling during the labeling phase, which is the essential phase to estimate average turnover rates. It also allows better spreading of blood withdrawals with time, keeping the burden of blood sampling for patients relatively low. Second, prolonged exposure to labeling allows even cells with relatively slow turnover rates, such as naive T cells, to become sufficiently labeled to reliably estimate their turnover. Third, longer labeling gives recently produced, and hence labeled, cells ample time to appear in the blood, where most measurements are generally taken. Naturally, the shorter the labeling period, the less saturation of subpopulations is expected to occur, and the smaller the requirement for a multiexponential model. However, even during short-term labeling, saturation may already be present. Therefore, it may be good to always use the multiexponential model, both for short-term and long-term labeling periods. As long as label accrual reflects the average population turnover (before any signs of saturation), the multiexponential model will behave like a single-exponential model, and the fitting procedure will set the contribution of the extra exponential(s) to zero. It will hence yield the same average turnover rate.
Remarkably, 2 earlier studies have reported longer life span estimates for murine memory CD8+ T cells than the 20-day average that we found. Labeling experiments with 5-bromo-2'-deoxyuridine in thymectomized mice revealed a CD8+ effector/memory T-cell half-life of 63 days (corresponding to a life span of 91 days),18 and later Choo et al19 showed that adoptively transferred lymphocytic choriomeningitis virus–specific memory CD8+ T cells and bulk CD44hi T cells had an intermitotic time of ∼50 days. A major advantage of stable-isotope labeling is that one can study cell turnover under physiological circumstances, without affecting immune homeostasis. This could be different for the (more manipulative) approaches used in the earlier studies. More in agreement with our results is a study by Younes et al,20 who proposed that the CD4+ memory pool is heterogeneous, comprising both slowly dividing “authentic” antigen-experienced memory cells as well as rapidly dividing “memory-phenotype” cells that arise from an antigen-independent mechanism. The level of 5-bromo-2′-deoxyuridine incorporation that they measured in CD4+ (CD44hi) memory-phenotype cells corresponds to a CD4+ memory T-cell life span of 14 to 22 days.21 This estimate is very similar to our CD4+ memory T-cell life span estimate of 15 days.
In humans, the current best estimates are that memory CD4+ and CD8+ T cells live 164 and 157 days, respectively. Again, these estimates are considerably shorter than our previous estimates of 222 and 357 days for memory CD4+ and CD8+ T cells, respectively.10 Thus, discrepancies in the literature on human T-cell life span estimates10,13 may have, to a large extent, been caused by the use of single-exponential models, which led to overestimation of T-cell life spans in long-term labeling studies. We cannot exclude that other, yet unidentified factors may cause differences in life span estimates, such as an intrinsic difference between 2H2O and deuterated glucose, which may underlie the remaining differences between the estimated life spans of the 1-week deuterated glucose experiment and the 9-week 2H2O experiment (Figure 5B). The part of the variation that is due to the length of the labeling period has at least been resolved thanks to the use of a multiexponential model.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank David Ho and Hiroshi Mohri for sharing their published data, and Linda McPhee for comments on the manuscript.
This research was supported by the Landsteiner Foundation for Blood Transfusion Research (grant 0210); the Netherlands Organisation for Scientific Research grants 927.50.029, 917.96.350, and 016.096.350; and visitor’s grant 040.11.128); the Virgo Consortium (Netherlands Genomics Initiative, BB.000342.1); and the Research Council for Earth and Life Sciences, with financial aid from the Netherlands Organisation for Scientific Research (grant 836.07.002).
Authorship
Contribution: L.W., J.D., B.A., R.J.d.B., K.T., and J.A.M.B. wrote the manuscript; L.W., I.d.B., and K.T. designed the experiments; L.W., I.d.B., L.K., T.V., E.H.R.v.d.W.-S., G.S., K.G., and M.T.A. performed the experiments; and J.D., T.M., I.v.d.M., I.B., R.J.d.B., and J.A.M.B. performed mathematical modeling.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: J.A.M. Borghans, Laboratory for Translational Immunology, University Medical Center Utrecht, WKZ Room KC 02.085.2, PO Box 85090, 3508 AB Utrecht, The Netherlands; e-mail: J.Borghans@umcutrecht.nl.
References
Author notes
L.W., J.D., and I.d.B. contributed equally to this study.
R.J.d.B., K.T., and J.A.M.B. contributed equally to this study.