Key Points
Peripheral B-cell tolerance is defective in IPEX patients, suggesting that Tregs are involved in the maintenance of B-cell tolerance.
T cells, including Tregs, display an activated phenotype in IPEX patients that may favor the accumulation of autoreactive B cells.
Abstract
Regulatory T cells (Tregs) play an essential role in preventing autoimmunity. Mutations in the forkhead box protein 3 (FOXP3) gene, which encodes a transcription factor critical for Treg function, result in a severe autoimmune disorder and the production of various autoantibodies in mice and in IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) patients. However, it is unknown whether Tregs normally suppress autoreactive B cells. To investigate a role for Tregs in maintaining human B-cell tolerance, we tested the reactivity of recombinant antibodies isolated from single B cells isolated from IPEX patients. Characteristics and reactivity of antibodies expressed by new emigrant/transitional B cells from IPEX patients were similar to those from healthy donors, demonstrating that defective Treg function does not impact central B-cell tolerance. In contrast, mature naive B cells from IPEX patients often expressed autoreactive antibodies, suggesting an important role for Tregs in maintaining peripheral B-cell tolerance. T cells displayed an activated phenotype in IPEX patients, including their Treg-like cells, and showed up-regulation of CD40L, PD-1, and inducibl T-cell costimulator (ICOS), which may favor the accumulation of autoreactive mature naive B cells in these patients. Hence, our data demonstrate an essential role for Tregs in the establishment and the maintenance of peripheral B-cell tolerance in humans.
Introduction
Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome is a primary immunodeficiency with severe autoimmunity caused by mutations in the forkhead box protein 3 (FOXP3) gene.1,2 A similar phenotype is observed in scurfy mice that carry a mutation in their foxp3 gene. FOXP3 encodes a transcription factor essential for the function of natural thymus-derived CD4+CD25+FOXP3+ regulatory T cells (Tregs), which in turn are critically important in maintaining self-tolerance in both mice and humans.3-5 Multiple and diverse autoantibodies are commonly identified in the sera of IPEX patients, suggesting that Tregs may represent a key regulator for autoreactive B cells.6
Antibodies are generated during early B-cell development by random joining of immunoglobulin (Ig) gene segments and therefore can result in the assembly of autoreactive antibodies or B-cell receptors (BCRs). It has been previously demonstrated that most developing autoreactive B cells in humans are removed at 2 discrete steps.7 First, a central checkpoint in the bone marrow between early immature and immature B cells removes the majority of developing B cells that express highly polyreactive antibodies and only a small fraction of clones with low levels of polyreactivity migrate to the periphery. Then, a peripheral B-cell tolerance checkpoint further counterselects autoreactive new emigrant B cells before they enter the mature naive B-cell compartment.7
The regulation of central B-cell tolerance in humans seems to be mostly controlled by B cell–intrinsic factors, which potentially include self-antigen binding receptors such as BCRs and Toll-like receptors (TLRs).8-11 Relatively less is known about the mechanisms that control the peripheral B-cell tolerance checkpoint in humans. The analysis of CD40L- and MHC class II–defective patients demonstrated that while developing autoreactive B cells are properly counterselected in the bone marrow in these patients, their mature naive B cells express a high proportion of autoreactive antibodies, including antinuclear antibodies (ANAs).12 These findings strongly supported the idea that a CD4+ T-cell population requiring CD40L and potentially self-antigen presentation through MHC class II likely prevent the accumulation of autoreactive B cells in the periphery. Interestingly, CD40L-deficient patients display reduced frequencies of CD4+CD25+CD127loFOXP3+ Tregs as well as elevated serum concentration of B-cell activating factor (BAFF) in their peripheral blood, providing indirect evidence for an important role of Tregs and/or serum BAFF in maintaining peripheral B-cell tolerance.12
To determine the impact of Tregs on the establishment of human early B-cell tolerance checkpoints, we cloned and expressed in vitro recombinant antibodies from single B cells from IPEX patients, and compared their reactivity to those derived from healthy donors. We report herein that FOXP3 defiency results in the accumulation of autoreactive clones in the mature naive B-cell compartment of IPEX patients, providing direct evidence for the role of Tregs in maintaining peripheral B-cell tolerance in humans.
Methods
Patients
IPEX patients' information is included in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Healthy donors were previously reported.7,8,10-12 All samples were collected in accordance with institutional review board–approved protocols and the Declaration of Helsinki.
Cell staining and sorting, cDNA, RT-PCR, antibody production, ELISAs, and indirect fluorescence assays
Peripheral B cells were purified from venous blood of patients and control donors by positive selection using CD20-magnetic beads (Miltenyi Biotec). Single CD19+CD21loCD10+IgMhiCD27− new emigrant/transitional and CD19+CD21+CD10−IgM+CD27− peripheral mature naive B cells from patients and control donors were sorted on a FACSAria (BD Biosciences) into 96-well PCR plates, and antibody reactivities were tested as previously described.7 Serum BAFF concentrations were determined by ELISA according to the manufacturer's instruction (R&D Systems). Flow cytometric stainings were performed using antibodies reported in supplemental Table 2. Intracellular staining for FOXP3 Alexa Fluor 488 (clone PCH101; eBioscience), Helios Alexa Fluor 647, and Ki67 PE (Biolegend) were performed using the Foxp3/Transcription Factor Staining Buffer Set (eBioscience).
KREC assay
The ratio of κ-deletion recombination excision circle (KREC) joints (signal joint) to the Jκ-Cκ recombination genomic joints (coding joint) was determined as previously described.13 Two separate real-time quantitative PCR reactions were performed, one reaction to amplify the signal joint and the other to amplify the coding joint, as previously detailed.13 The number of cell divisions was calculated by subtracting the cycle threshold of the PCR detecting the coding joint from that of the PCR detecting the signal joint.
Real-time RT-PCR analysis
Total RNA from CD20-depleted PBMCs was extracted using the RNeasy Kit (QIAGEN) and 150 ng of RNA samples were reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad). The resulting cDNA was amplified in duplicate using primer sets reported in supplemental Table 3, Brilliant SYBR Green QPCR Master Mix (Agilent), and the Stratagene MX3005 real-time detection system. The results were normalized to ACTIN for each sample before comparisons between IPEX patients and healthy controls.
Statistical analysis
Differences between patients and healthy donors were analyzed for statistical significance with unpaired Student t tests, using GraphPad Prism software.
Results
Central B-cell tolerance is functional in IPEX patients
FOXP3 gene expression is restricted to the T-cell lineage and defines regulatory T-cell populations but may also be transiently expressed after T-cell activation in humans.14-17 Mutations in the FOXP3 gene from patients with the IPEX syndrome affect the function of CD4+CD25+CD127loFOXP3+ Tregs and possibly impinge on effector CD4+ T-cell function.1,18,19 While we and others have found that bone marrow B-cell precursors and peripheral blood B cells do not express FOXP3 (data not shown),14,20 the impact of FOXP3 deficiency on B-cell development and selection has not been properly assessed.
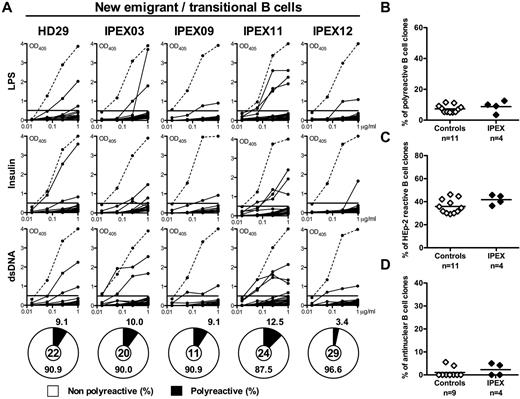
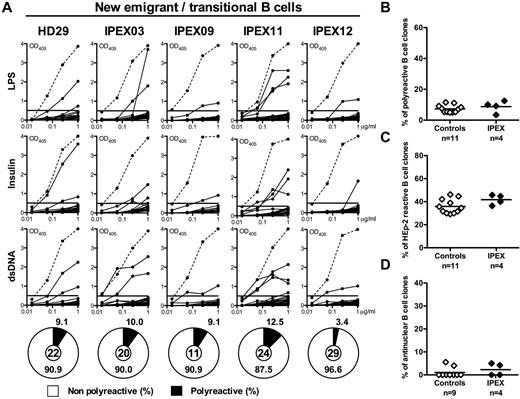
To determine whether human B-cell development and early B-cell tolerance checkpoints are affected by the absence of functional FOXP3, we analyzed B-cell development in 10 IPEX patients with different FOXP3 mutations and tested the reactivity of recombinant antibodies cloned from 84 single CD19+CD21lo CD10+IgMhiCD27− new emigrant/transitional B cells and 97 CD19+CD21+CD10−IgM+CD27− mature naive B cells (sorting strategy shown in supplemental Figure 1) from 4 IPEX patients (Figure 1). Three of these IPEX patients were on low-dose immunosuppressive regimens, whereas 1 IPEX patient (IPEX12) was naive to any medication because his blood sample was obtained before any suppressive or conditioning regimen was started. All B-cell subpopulations were present within normal ranges of age-matched control donors, suggesting that FOXP3 deficiency does not appear to result in gross B-cell development defects (supplemental Figure 1 and data not shown). Moreover, immunoglobulin heavy and light chain gene segment usage was similar in new emigrant/transitional B cells from healthy donors and IPEX patients, including heavy chain D reading frame selection (supplemental Figure 2, supplemental Tables 4-9). In addition, the frequencies of new emigrant/transitional polyreactive B cells ranged between 3.4% and 12.5% in IPEX patients and were comparable with those previously reported in healthy donors (5.0%-11.1%; Figure 1A-B), suggesting that central B-cell tolerance is properly established in the absence of functional FOXP3. Interestingly, there were no differences between the treatment naive-IPEX12 patient and the other subjects, suggesting that the immunosuppressive treatment does not seem to grossly affect B-cell selection (Figure 1). Moreover, the frequencies of HEp-2– and antinuclear-reactive new emigrant/transitional B cells from IPEX patients were also similar to those in healthy donors, further demonstrating the proper removal of developing autoreactive B cells in the bone marrow of these patients (Figure 1C-D, supplemental Figure 3). We conclude that the absence of functional Tregs in IPEX patients does not interfere with central B-cell tolerance in humans.
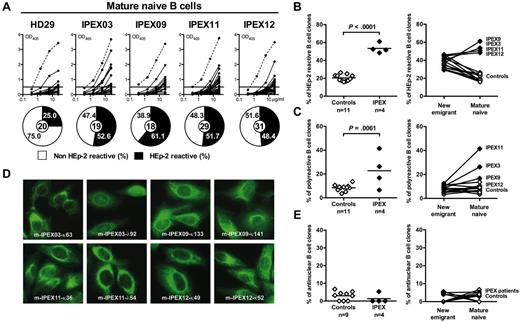
The central B-cell tolerance checkpoint is functional in IPEX patients. (A) Antibodies from new emigrant/transitional B cells from a healthy donor and IPEX patients were tested by ELISA for reactivity against dsDNA, insulin, and lipopolysaccharide (LPS). Dotted lines show ED38-positive control and solid lines show binding for each cloned recombinant antibody. Horizontal lines define cutoff OD405 for positive reactivity. For each individual, the frequency of polyreactive (filled area) and nonpolyreactive (open area) clones is summarized in pie charts, with the total number of clones tested indicated in the centers. The frequencies of (B) polyreactive, (C) HEp-2–reactive, and (D) antinuclear new emigrant/transitional B cells in healthy controls and IPEX patients are shown.
The central B-cell tolerance checkpoint is functional in IPEX patients. (A) Antibodies from new emigrant/transitional B cells from a healthy donor and IPEX patients were tested by ELISA for reactivity against dsDNA, insulin, and lipopolysaccharide (LPS). Dotted lines show ED38-positive control and solid lines show binding for each cloned recombinant antibody. Horizontal lines define cutoff OD405 for positive reactivity. For each individual, the frequency of polyreactive (filled area) and nonpolyreactive (open area) clones is summarized in pie charts, with the total number of clones tested indicated in the centers. The frequencies of (B) polyreactive, (C) HEp-2–reactive, and (D) antinuclear new emigrant/transitional B cells in healthy controls and IPEX patients are shown.
Defective peripheral B-cell tolerance checkpoint in IPEX patients
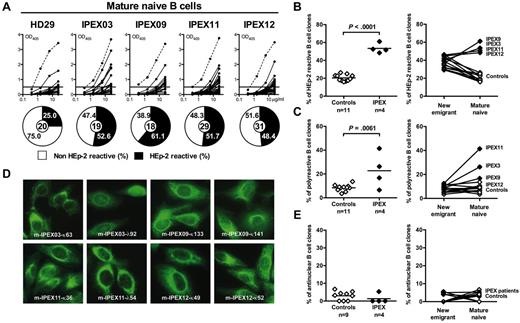
To analyze the effect of FOXP3-mutated dysfunctional Tregs on the peripheral B-cell tolerance checkpoint, we analyzed the immunoglobulin repertoire and tested the reactivity of recombinant antibodies from mature naive B cells from IPEX patients against HEp-2 cell lysates using ELISA (Figure 2A). Striking differences in reading frame selection for most heavy chain D segments were observed in mature naive B cells from IPEX patients (supplemental Figure 2, supplemental Tables 10-15). Contrasting with healthy donor's mature naive B cells, hydrophobic D reading frames, which favor antibody self-reactivity,8 were commonly used in IPEX mature naive B cells (supplemental Figure 2). Correlating with these abnormal immunoglobulin repertoire features, we found that the frequency of mature naive B cells that expressed HEp-2–reactive antibodies was significantly increased in all 4 IPEX patients (48.4%-61.1%) compared with the healthy donors (16.7%-26.3%, P < .001; Figure 2A-B). In addition, the frequency of mature naive polyreactive B cells was higher in IPEX patients compared with healthy donors (P = .0061), and increased between the new emigrant/transitional and mature naive compartments in all patients (Figure 2C, supplemental Figure 4). Interestingly, despite the high frequency of HEp-2– and polyreactive mature naive B cells in IPEX patients, we did not observe any increase in antinuclear clones in the mature naive B-cell compartment of these patients in contrast to CD40L- and activation-induced cytidine deaminase (AID)–deficient patients who also displayed an abnormal peripheral B-cell tolerance checkpoint (Figure 2D-E).12,21 Hence, the absence of functional Tregs results in a defective peripheral B-cell tolerance checkpoint with an increase in B cells expressing HEp-2–reactive and polyreactive, but not antinuclear, antibodies.
Defective peripheral B-cell tolerance checkpoint in IPEX patients. (A) Antibodies from mature naive B cells from a healthy donor and IPEX patients were tested by ELISA for anti-HEp-2 cell reactivity. Dotted lines show ED38-positive control and solid lines show binding for each cloned recombinant antibody. Horizontal lines define cutoff OD405 for positive reactivity. For each individual, the frequency of HEp-2–reactive (filled area) and non-HEp-2–reactive (open area) clones is summarized in pie charts, with the total number of clones tested indicated in the centers. The frequencies of (B) HEp-2–reactive and (C) polyreactive mature naive B cells in healthy controls and IPEX patients (left) and their evolution between the new emigrant/transitional and mature naive B-cell compartments (right) are shown. (D) Mature naive B-cell clones from IPEX patients show various patterns of cytoplasmic HEp-2 staining. (E) The frequency of antinuclear clones is low (left) and is not increased between the new emigrant/transitional and mature naive B-cell compartments (right).
Defective peripheral B-cell tolerance checkpoint in IPEX patients. (A) Antibodies from mature naive B cells from a healthy donor and IPEX patients were tested by ELISA for anti-HEp-2 cell reactivity. Dotted lines show ED38-positive control and solid lines show binding for each cloned recombinant antibody. Horizontal lines define cutoff OD405 for positive reactivity. For each individual, the frequency of HEp-2–reactive (filled area) and non-HEp-2–reactive (open area) clones is summarized in pie charts, with the total number of clones tested indicated in the centers. The frequencies of (B) HEp-2–reactive and (C) polyreactive mature naive B cells in healthy controls and IPEX patients (left) and their evolution between the new emigrant/transitional and mature naive B-cell compartments (right) are shown. (D) Mature naive B-cell clones from IPEX patients show various patterns of cytoplasmic HEp-2 staining. (E) The frequency of antinuclear clones is low (left) and is not increased between the new emigrant/transitional and mature naive B-cell compartments (right).
Normal serum BAFF concentrations in IPEX patients
BAFF is a critical B-cell survival factor that controls the number of peripheral B cells.22 In transgenic mouse models, increased BAFF levels interfere with the counterselection of autoreactive new emigrant/transitional B cells, which can reach the mature B-cell compartment.22
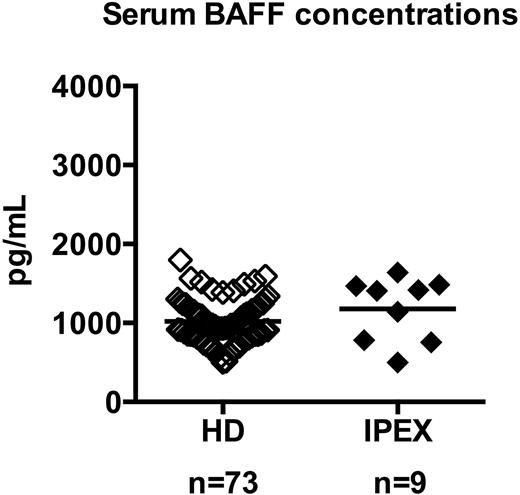
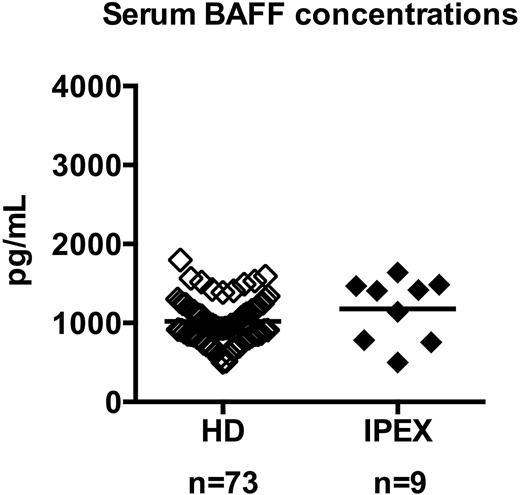
To determine whether serum BAFF may also account for the defective peripheral B-cell tolerance identified in IPEX patients, we measured by ELISA the serum BAFF concentration in 10 IPEX patients. IPEX01 was excluded from this analysis because he received anti–B-cell therapy, which resulted in a 10-time increase of his serum BAFF concentration linked to an absence of circulating B cells (not shown). Serum BAFF concentrations in the other 9 IPEX patients were within the normal range of healthy controls (Figure 3). Hence, the 4 IPEX patients who displayed a defective peripheral B-cell tolerance checkpoint showed normal serum BAFF concentrations, demonstrating that these defects are not likely to result from dysregulated serum BAFF concentrations.
IPEX patients display normal serum BAFF concentrations. Serum BAFF concentrations (picograms per milliliter) in healthy donors (♢, n = 73) and IPEX patients (♦, n = 9) were measured by ELISA. Each diamond represents an individual, and the average is shown with a bar: healthy donors, 1019 ± 35; IPEX patients, 1177 ± 134.
IPEX patients display normal serum BAFF concentrations. Serum BAFF concentrations (picograms per milliliter) in healthy donors (♢, n = 73) and IPEX patients (♦, n = 9) were measured by ELISA. Each diamond represents an individual, and the average is shown with a bar: healthy donors, 1019 ± 35; IPEX patients, 1177 ± 134.
Mature naive B cells from IPEX patients show limited activation but increased homeostatic expansion
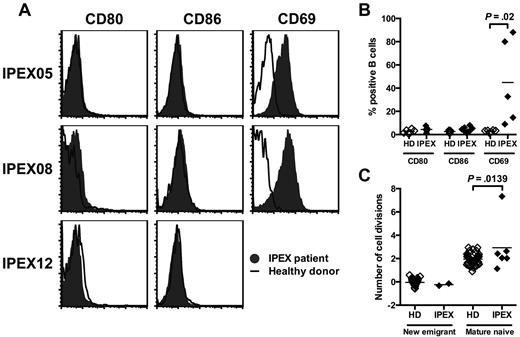
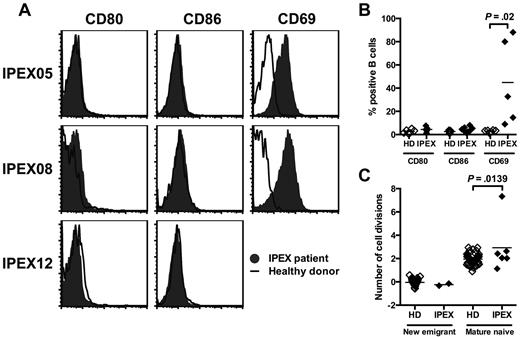
In an attempt to uncover the mechanisms by which Tregs may prevent the accumulation of autoreactive B cells in the periphery, we analyzed the phenotype of B cells in IPEX patients. In contrast to Foxp3-deficient mice,20 we did not observe any up-regulation of CD80 and CD86 on mature naive B cells from any of the 7 analyzed IPEX patients, including the treatment naive IPEX patient, suggesting that the absence of CD80 and CD86 expression on IPEX B cells was not because of their immunosuppressive regimen (Figure 4A-B). We did not detect any change in IL-2Rα/CD25 expression (data not shown), but CD69 was up-regulated on B cells from all IPEX patients (Figure 4A-B). Hence, the absence of functional Tregs in IPEX patients seems to only result in a moderate B-cell activation in the periphery. We further assessed whether Tregs affect peripheral B-cell homeostasis by analyzing the in vivo replication history of new emigrant/transitional and mature naive B-cell fractions by using the PCR-based assay for measuring KRECs, which gives an estimate on the number of divisions a sorted B-cell population has undergone.13 In agreement with previously published results, we observed that new emigrant/transitional B cells from healthy donors have no division history (0 division ± 0.3) whereas their mature naive B cells undergo homeostatic expansion in the periphery as illustrated by an average of 2.0 ± 0.5 divisions per B cells (Figure 4C).13 In contrast, the analysis of mature naive B-cell populations from IPEX patients reveals that these B cells have undergone on average one additional division in the absence of functional Tregs (Figure 4C). We conclude that FOXP3 deficiency in IPEX patients does not lead to extensive B-cell activation but may fail to prevent some homeostatic B-cell expansion, thereby suggesting that the increased frequency of autoreactive mature naive B cells in these patients may result from the preferential proliferation of autoreactive clones in the periphery.
Mature naive B cells from IPEX patients show an increased proliferative history. (A) Representative CD80, CD86, and CD69 expression on CD19+CD27− naive B cells in IPEX patients (solid gray) compared with healthy donors (bold). (B) Mean fluorescence intensities (MFI) of CD80, CD86, and CD69 expression on naive B cells from healthy donors (n = 7) and IPEX patients (n = 7). (C) Evaluation of the number of cell divisions undergone in vivo by KREC analysis on new emigrant and mature naive B cells of healthy donors (n = 41) and IPEX patients (n = 6).
Mature naive B cells from IPEX patients show an increased proliferative history. (A) Representative CD80, CD86, and CD69 expression on CD19+CD27− naive B cells in IPEX patients (solid gray) compared with healthy donors (bold). (B) Mean fluorescence intensities (MFI) of CD80, CD86, and CD69 expression on naive B cells from healthy donors (n = 7) and IPEX patients (n = 7). (C) Evaluation of the number of cell divisions undergone in vivo by KREC analysis on new emigrant and mature naive B cells of healthy donors (n = 41) and IPEX patients (n = 6).
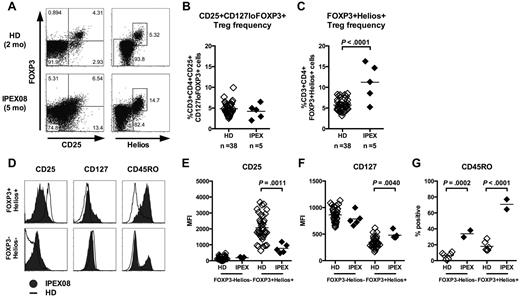
Increased frequency of CD4+FOXP3+Helios+ natural Treg-like cells in IPEX patients
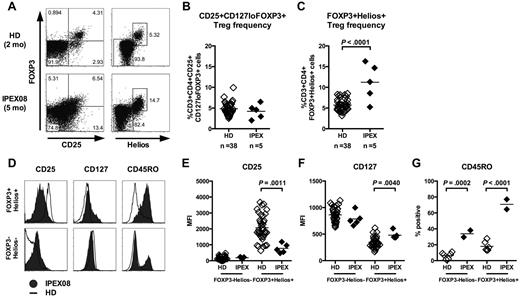
Most IPEX patients carry a missense FOXP3 mutation that allows FOXP3 protein expression but alters its function (supplemental Table 1). As a consequence, we found normal frequencies of CD3+CD4+CD25+CD127loFOXP3+ Treg-like cells in the peripheral blood of all 5 analyzed IPEX patients, as previously reported (Figure 5A-B, supplemental Figure 5A).23,24 To further analyze the Treg compartment in IPEX patients, we analyzed the expression of Helios, an Ikaros-family transcription factor suggested to be expressed by thymus-derived FOXP3+ Tregs.25 We observed a significantly elevated frequency of CD4+FOXP3+Helios+ T cells compared with healthy controls in 4 of 5 IPEX patients (Figure 5A,C, supplemental Figure 5A). In addition, almost all FOXP3+ Tregs were Helios+ in very young subjects, further suggesting that these Tregs were of thymic origin as initially postulated, whereas inducible Helios− Tregs could be identified in older patients and healthy donors (Figure 5, supplemental Figure 5).25 In healthy controls, CD4+FOXP3+Helios+ T cells had the typical Treg phenotype characterized by the high expression of IL-2Rα/CD25 and low expression of IL-7Rα/CD127 (Figure 5D, supplemental Figure 5B). However, Tregs from IPEX patients have down-regulated CD25 and up-regulated CD127 expression, a phenotype typically associated with the loss of FOXP3 (Figure 5D-F).26,27 Hence, increased Treg frequencies in IPEX patients may have been missed when using the classic CD4+CD25+FOXP3+ Treg gating strategy. In addition, we also observed an increased expression of the memory marker CD45RO on CD4+FOXP3+Helios+ T cells from the youngest IPEX patients (IPEX05 and IPEX08) compared with age-matched healthy controls (Figure 5D,G, supplemental Figure 5B). Indeed, only 13% to 27% Tregs from healthy 0- to 3-year-old infants expressed CD45RO and belonged to the memory T-cell compartment whereas 65% to 77% of Tregs in young IPEX patients were CD45RO+, suggesting that Treg-like cells from IPEX patients have already been activated in vivo even at such young age (Figure 5G, supplemental Figure 5B). This notion was also supported by increased expression of the proliferation marker Ki67 by CD4+FOXP3+Helios+ T cells from these IPEX patients (supplemental Figure 6). Hence, our data suggest that the thymus-derived Treg population defined as CD4+FOXP3+Helios+ is expanded in the peripheral blood of IPEX patients and is not capable of maintaining its regulatory T-cell phenotype and function.
Increased frequency of CD4+FOXP3+Helios+ T cells in IPEX patients. (A) Representative CD25 and FOXP3 (left) and Helios and FOXP3 staining (right) on CD3+CD4+ cells from a healthy donor and a representative IPEX patient. CD3+CD4+CD25+CD127loFOXP3+ (B) and CD3+CD4+FOXP3+Helios+ (C) T-cell frequencies in 38 healthy donors and 5 IPEX patients. (D) Expression levels of CD25, CD127, and CD45RO on FOXP3−Helios− and FOXP3+Helios+ CD4+ T cells from a representative IPEX patient (solid gray) and a healthy donor (bold) gated as shown in panel A. The mean fluorescence intensities (MFI) of (E) CD25, (F) CD127, and (G) CD45RO expression is shown for Helios−FOXP3− and Helios+FOXP3+ CD4+ T cells from healthy donors and IPEX patients.
Increased frequency of CD4+FOXP3+Helios+ T cells in IPEX patients. (A) Representative CD25 and FOXP3 (left) and Helios and FOXP3 staining (right) on CD3+CD4+ cells from a healthy donor and a representative IPEX patient. CD3+CD4+CD25+CD127loFOXP3+ (B) and CD3+CD4+FOXP3+Helios+ (C) T-cell frequencies in 38 healthy donors and 5 IPEX patients. (D) Expression levels of CD25, CD127, and CD45RO on FOXP3−Helios− and FOXP3+Helios+ CD4+ T cells from a representative IPEX patient (solid gray) and a healthy donor (bold) gated as shown in panel A. The mean fluorescence intensities (MFI) of (E) CD25, (F) CD127, and (G) CD45RO expression is shown for Helios−FOXP3− and Helios+FOXP3+ CD4+ T cells from healthy donors and IPEX patients.
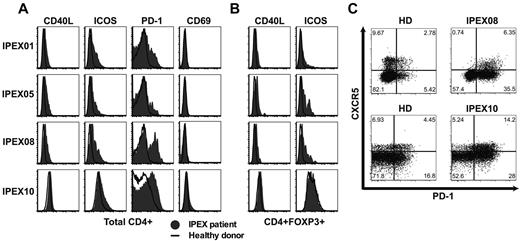
IPEX patients display circulating T follicular helper–like cells in their blood
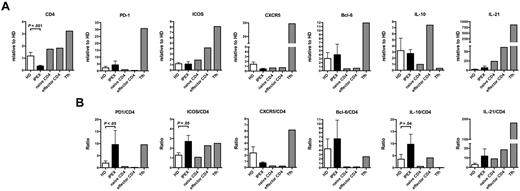
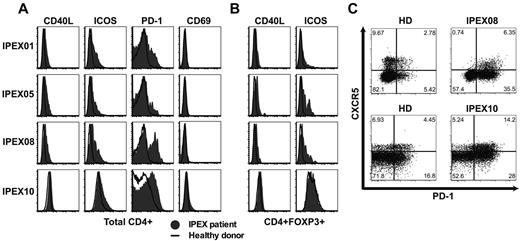
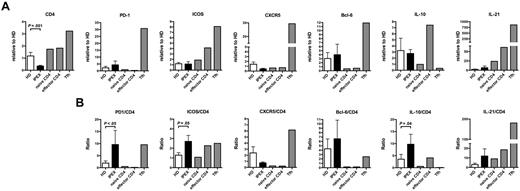
Tregs play an essential role in maintaining immune homeostasis and preventing the activation of effector T (Teff) cells. To assess how the absence of functional Tregs affects the other T-cell compartments, we analyzed the expression of activation and costimulatory molecules on CD4+ T cells from IPEX patients. We consistently observed the up-regulation of CD40L, PD-1, and inducible T-cell costimulator (ICOS) but not CD69 on total CD4+ and even enhanced in CD45RO+ memory T cells from IPEX patients, revealing a global activation of CD4+ T cells in the absence of Treg repression (Figure 6A, supplemental Figure 7). CD40L expression was also higher in the CD4+CD25+CD127loFOXP3+ Treg-like subset from IPEX patients, further demonstrating that these T cells were also activated in the absence of FOXP3-suppressive activity (Figure 6B). Peripheral blood CD4+CXCR5+ T cells, which are thought to represent the circulating pool of memory T follicular helper (Tfh) cells in humans,28 expressed higher levels of PD-1 and ICOS in IPEX patients, demonstrating an activated state normally characteristic of Tfh in germinal centers (Figure 6C).29,30 We further analyzed the presence of Tfh-like cells in the blood of IPEX patients by measuring transcript levels characteristic of this lineage as well as other T-cell subpopulations in B cell–depleted peripheral blood mononuclear cells (PBMCs; Figure 7). We found that PD1, BCL6, and IL21 transcripts were modestly increased in the PBMCs of IPEX patients who displayed significantly decreased CD4 transcripts, whereas they were highly expressed in positive control Tfh cells isolated from human tonsils (Figure 7A). Because Tfh cells express CD4, we analyzed these transcripts levels in comparison to CD4 and detected significant increases of PD1 and ICOS in IPEX patients (Figure 7B). BCL6 and IL21 transcripts were on average 2- to 3-fold increased in IPEX patients compared with healthy donors, but these differences failed to reach statistical significance, likely because of the small number of patients analyzed in this assay (Figure 7B). Significantly increased IL10 transcripts when normalized to CD4 may correspond to the higher frequency of Treg-like and Tr1 cells observed in IPEX patients (Figure 7B).31 These T cells are known to secrete this cytokine but the cell origin of these transcripts remains to be determined. We conclude that the higher frequency of circulating CD4+CXCR5+PD-1+ICOS+ T cells, combined with increased levels of gene transcripts normally found in Tfh cells, suggests that Tfh-like cells are enriched in the blood of IPEX patients.
IPEX patients harbor activated T cells including Tfh-like cells. Increased expression of CD40L, ICOS, and PD-1 but not CD69 on total (A) CD3+CD4+ cells and (B) CD3+CD4+FOXP3+ cells in IPEX patients (solid gray) compared with healthy donors (bold). (C) Increased PD-1 expression on circulating CD4+CXCR5+ cells reveals Tfh-like cells in IPEX patients.
IPEX patients harbor activated T cells including Tfh-like cells. Increased expression of CD40L, ICOS, and PD-1 but not CD69 on total (A) CD3+CD4+ cells and (B) CD3+CD4+FOXP3+ cells in IPEX patients (solid gray) compared with healthy donors (bold). (C) Increased PD-1 expression on circulating CD4+CXCR5+ cells reveals Tfh-like cells in IPEX patients.
PBMCs from IPEX patients reflect the presence of Tfh-like cells. (A) Quantitative real-time PCRs validate the increased transcription of Tfh genes in IPEX patients. Gene expression was assessed by comparing 11 healthy donors and 5 IPEX patients. Error bars represent the mean ± SEM. (B) Gene expression is displayed relative to CD4 expression and demonstrates PD1, ICOS, and IL10 up-regulation in IPEX patients.
PBMCs from IPEX patients reflect the presence of Tfh-like cells. (A) Quantitative real-time PCRs validate the increased transcription of Tfh genes in IPEX patients. Gene expression was assessed by comparing 11 healthy donors and 5 IPEX patients. Error bars represent the mean ± SEM. (B) Gene expression is displayed relative to CD4 expression and demonstrates PD1, ICOS, and IL10 up-regulation in IPEX patients.
Discussion
We report herein that IPEX patients carrying a FOXP3 gene mutation suffer from specific defects in their peripheral B-cell tolerance checkpoint, resulting in the accumulation of large numbers of autoreactive mature naive B cells in their blood. In contrast, we found that the frequency of autoreactive, polyreactive, and antinuclear new emigrant/transitional B cells in these patients was comparable with that in healthy controls, demonstrating that the central B-cell tolerance checkpoint is functional in IPEX patients. Therefore, the absence of functional Tregs does not appear to affect the counterselection of autoreactive immature B cells in the bone marrow. In agreement with these results, deficiencies in CD40L expressed by T cells and MHC class II allowing antigen presentation to T cells do not impact central B-cell tolerance in humans.12 Hence, interactions with T cells and especially Tregs are dispensable for the removal of developing autoreactive B cells in the bone marrow, which seems to be regulated by intrinsic B-cell factors.8-11
CD40L-, MHC class II–deficient, as well as AID-deficient patients showing a defective peripheral B-cell tolerance checkpoint displayed lower numbers of CD4+CD25+CD127loFOXP3+ Tregs, providing indirect evidence for an involvement of these T cells in the establishment and/or the maintenance of peripheral B-cell tolerance.12,21 However, patients with these defects also displayed elevated BAFF concentrations in their serum, which may directly interfere with the counterselection of peripheral autoreactive B cells.22 The finding that IPEX patients with a defective peripheral B-cell tolerance checkpoint, displayed normal serum BAFF concentration, suggests that Treg function rather than high BAFF concentrations control the peripheral B-cell tolerance checkpoint in humans. The increase in naive B cells expressing ANAs in CD40L- and AID-deficient but not in IPEX patients also suggests that elevated serum BAFF concentrations may contribute to defective peripheral B-cell tolerance potentially by favoring the survival of antinuclear clones.12,21 In line with this observation is the fact that autoantibody and ANA production is observed frequently in systemic lupus erythematosus (SLE), Sjögren syndrome, as well as in GVHD who have been reported to display elevated serum BAFF concentrations.22
How can defective Treg functions result in a defective peripheral B-cell tolerance checkpoint? It has been previously proposed that T/B-cell interactions may play an important role in the counterselection of autoreactive B cells in the periphery.32 Our results are in agreement with this model and further demonstrate an important role for Tregs in counterselecting autoreactive B cells in humans. A critical role for Tregs in maintaining B-cell tolerance has also been revealed with several mouse models.33-35
However, it is unclear whether Tregs maintain B-cell tolerance through direct interactions with autoreactive B cells, or indirectly, for example, by suppressing Teff cells.35 A direct mechanism is supported by the finding that activated mouse Tregs can selectively induce apoptosis of autoreactive B cells in vitro.36 Activated Tregs were also shown in vitro to induce apoptosis of B cells from SLE patients but not of those from healthy controls, suggesting that this phenomenon was linked to B-cell hyperactivity.37 Alternatively, Tregs block Teff cells from providing help to autoreactive B cells in a murine model of lupus.38 Nonetheless, our data suggest that both Treg and Teff cells from IPEX patients display an activated phenotype similar to that of CD4+ T cells derived from foxp3-deficient mice and characterized by the up-regulation of many costimulatory molecules such as CD40L, PD-1, and ICOS, which may provide survival signals to new emigrant/transitional B cells and favor their survival in the mature naive B-cell compartment of IPEX patients.39 However, the analysis of CD40L-deficient patients with a defective peripheral B-cell tolerance checkpoint revealed that CD40/CD40L interactions are not required for the survival of autoreactive B cells, pointing to an important role for other costimulatory molecules such as perhaps PD-1/PD-1 ligands and ICOS/ICOSL.12 Recently, Tregs have been shown to be able to suppress Tfh cells. Indeed, Tregs can adopt a Tfh differentiation program and express the follicular homing receptor CXCR5, which allows their localization to germinal centers.40,41 These T follicular regulator cells limit germinal center B-cell numbers probably through the suppression of Tfh cells.40,41 In humans, FOXP3+ T cells have been observed in germinal centers and shown to suppress the production of antibodies by B cells.42 Our data obtained in IPEX patients support the hypothesis that Tregs suppress Tfh cells because in the absence of functional Tregs, Tfh-like cells circulate in the blood of IPEX patients. Because Tfh cells normally provide B-cell help in germinal centers, we propose that these T cells are likely to favor autoreactive B-cell survival through either cell-cell interactions or by the secretion of cytokines such as IL-21.
By following the coexpression of FOXP3 and Helios transcription factors, which may define natural, thymus-derived Tregs,25 we found that many IPEX patients actually displayed an increased frequency of CD4+FOXP3+Helios+ T cells. However, these T cells expressed reduced levels of IL-2Rα/CD25 and higher levels of IL-7Rα/CD127 in IPEX patients. Because FOXP3 has been shown to bind to the promoter of the IL2RA/CD25 gene,43,44 our finding may reflect the inability of mutant FOXP3 proteins from IPEX patients to up-regulate CD25 expression in Tregs, as previously demonstrated with transduction experiments.27 In addition, we observed that many CD4+FOXP3+Helios+ Treg-like cells from IPEX patients who were only a few months old already expressed the memory marker CD45RO and the proliferation molecule Ki67 whereas few Tregs from age-matched controls were found positive for these markers, suggesting that these CD4+FOXP3+Helios+ T cells with nonfunctional FOXP3 protein were specifically activated in vivo as previously reported in foxp3-deficient mice.39 It is conceivable that a TCR-induced proliferation of Tregs in IPEX patients may explain the increased frequency of CD4+FOXP3+ Helios+ Tregs found in these patients. Our findings in IPEX patients are in full agreement with data obtained in an elegant mouse model in which the fate of “ExFoxp3” T cells that have lost Foxp3 expression can be monitored.26,45 Indeed, these ExFoxp3 CD4+ T cells had an activated memory phenotype, reduced CD25 and increased CD127 expression and the capacity to produce inflammatory cytokines and induce autoimmunity.26,45 Alternatively, we cannot rule out that CD4+CD25+CD127+FOXP3+ Helios+ T cells from IPEX patients do not belong to the Treg lineage but might correspond to an expanded and activated Teff population that express some FOXP3 and Helios.16,17,46
Treg-like cells may develop in IPEX patients in the absence of functional FOXP3. However, these Tregs have a decreased capacity to suppress Teff cells in vitro.18,24 The activated T-cell phenotype and the expansion of both Treg-like and Tfh-like cells in IPEX patients observed in this study provide further in vivo evidence to support this hypothesis. In addition to the abnormal suppressive capacity of Tregs from IPEX patients, several indirect lines of evidence suggest that FOXP3 deficiency may also lead to the conversion of autoreactive Tregs to a Teff phenotype, further contributing to the pathogenesis of IPEX.15 This notion is supported by the recent findings demonstrating increased IL-17 production in IPEX patients,19 a phenomenon that may be driven, at least in part, by the conversion of dysfunctional Tregs into inflammatory effector cells.
Our findings may also have implications for the pathogenesis of common autoimmune diseases, such as rheumatoid arthritis (RA), type 1 diabetes (T1D), and multiple sclerosis, in which multiple defects in Treg function have been reported.47-49 By analogy to IPEX patients, these Treg defects may explain the defective peripheral B-cell tolerance checkpoint and the accumulation of autoreactive B-cell clones in the mature naive B-cell compartment already reported in RA and T1D.9,50
In conclusion, we propose that the defective peripheral B-cell tolerance checkpoint in IPEX patients is caused by dysfunctional Tregs that fail to control the activation of autoreactive CD4+ T cells potentially including Tfh cells, consequently leading to the preferential selection of autoreactive B cells. Alternatively, because of the phenotypic instability of these Tregs in the absence of functional FOXP3, it is possible that they directly act on autoreactive B cells either by failing to suppress them, or even by providing them survival and activation signals through self-antigen recognition presented by MHC class II. These 2 scenarios do not have to be mutually exclusive but can rather collectively contribute to the observed defect in peripheral B-cell tolerance in IPEX patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr S. Rudchenko and L. Devine for cell sorting and Jason Bannock and Christopher Massad for technical assistance.
This work was supported by grant nos. AI061093, AI071087, AI082713, and AI095848 from the National Institutes of Health–National Institute of Allergy and Infectious Diseases (NIH-NIAID; E.M.). T. K. was supported by the Sigrid Juselius Foundation, the Finnish Medical Foundation, and the Saastamoinen Foundation. H.M. received support from the German Research Foundation (DFG, MO 2160/2-1).
National Institutes of Health
Authorship
Contribution: T.K., N.C., H.M., J. Choi, S.K., and J. Craft performed the experiments and analyzed the data; T.K. and E.M. designed the research and wrote the manuscript; and E.M. initiated the collaboration with L.M., C.C., L.P., R.B., H.D.O., and T.R.T who provided patient blood samples and provided comments on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for T.K. is Department of Clinical Microbiology, University of Eastern Finland, Kuopio, Finland.
Correspondence: Eric Meffre, Yale University School of Medicine, 300 George St, New Haven, CT 06511; e-mail: eric.meffre@yale.edu.