Abstract
Pediatric stroke is a rare but highly penetrant disease with a strong genetic background. Although there are an increasing number of genome-wide association studies (GWASs) for stroke in adults, such studies for stroke of pediatric onset are lacking. Here we report the results of the first GWAS on pediatric stroke using a large cohort of 270 family-based trios. GWAS was performed using the Illumina 370 CNV single nucleotide polymorphisms array and analyzed using the transmission disequilibrium test as implemented in PLINK. An enrichment analysis was performed to identify additional true association signals among lower P value signals and searched for cumulatively associated genes within protein interaction data using dmGWAS. We observed clustering of association signals in 4 genes belonging to one family of metalloproteinases at high (ADAMTS12, P = 2.9 × 10−6; ADAMTS2, P = 8.0 × 10−6) and moderate (ADAMTS13, P = 9.3 × 10−4; ADAMTS17, P = 8.5 × 10−4) significance levels. Over-representation and gene-network analyses highlight the importance of the extracellular matrix in conjunction with members of the phosphoinositide and calcium signaling pathways in the susceptibility for pediatric stroke. Associated extracellular matrix components, such as ADAMTS proteins, in combination with misbalanced coagulation signals as unveiled by gene network analysis suggest a major role of postnatal vascular injury with subsequent thrombus formation as the leading cause of pediatric stroke.
Introduction
Pediatric stroke (PS) is a heterogeneous disorder associated with significant morbidity and mortality. It is recognized as an important childhood disease, with an incidence of 2.6-6.4 per 100 000 children per year.1 Although PS is relatively rare, it is devastating to those affected as half of the survivors develop cognitive or motor disabilities.2
Risk factors for stroke are different in children and adults, and classic risk factors, such as smoking, arteriosclerosis, or diabetes, are unlikely to contribute to pediatric stroke. The most prominent risk factors for stroke in children include underlying medical conditions (ie, cardiac disorders, metabolic diseases, cerebrovascular pathologies, and infectious diseases)1 as well as many prothrombotic abnormalities.3,4
Numerous association studies have established several genetic polymorphisms contributing to pediatric stroke risk. These candidate gene approaches identified several susceptibility genes for PS (ie, prothrombin, factor V-Leiden, and GPX3).3,4
However, genetic predisposition, environmental effects, and other risk factors can often not be disentangled because of the complex nature of PS etiology. Furthermore, a large proportion of missing heritability remains to be accounted for.5
Genome-wide association studies (GWASs) offer a powerful approach to gene function discovery and are the current method of choice to dissect the genetic basis of complex diseases. Up-to-date, studies in families with a known first onset of pediatric stroke are lacking mainly because of limited sample size.
Therefore, we performed a family-based GWAS for pediatric stroke in 270 families from Muenster, Germany composed of affected children and their parents. We identified several single nucleotide polymorphisms (SNPs), which are associated with PS at significance levels P < 10−5. In addition, we determined potential combined effects of SNPs contributing to stroke risk that may be missed through conventional single marker or haplotype association. We assessed the complete GWAS dataset under a systems biology aspect incorporating protein interaction information to gain further insight into the biologic networks underlying pediatric stroke.6 The latter analysis highlights the complex interplay of genes at the interface of vascular biology and coagulation in the pathogenesis of PS and supports the need of an extensive analysis of combined genetic and physiologic properties of complex diseases.
Methods
The study was approved by the medical ethics committee of the University of Muenster (Muenster, Germany) and complies with the ethical standards established in the updated version of the 1964 Declaration of Helsinki. Written informed consent was given from both parents for all families enrolled.
Subjects
Patients were enrolled between 2000 and 2009 as part of a nationwide survey (Erhebungseinheit für seltene pädiatrische Erkrankungen in Deutschland [ESPED] registry) and centrally documented in the Muenster pediatric stroke database. Stroke subtypes were reclassified according to explicit predefined criteria based on the TOAST criteria modified for children, by substituting arteriopathy for large-vessel atherosclerosis, by an independent blinded team of neuroradiologists (for details see supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
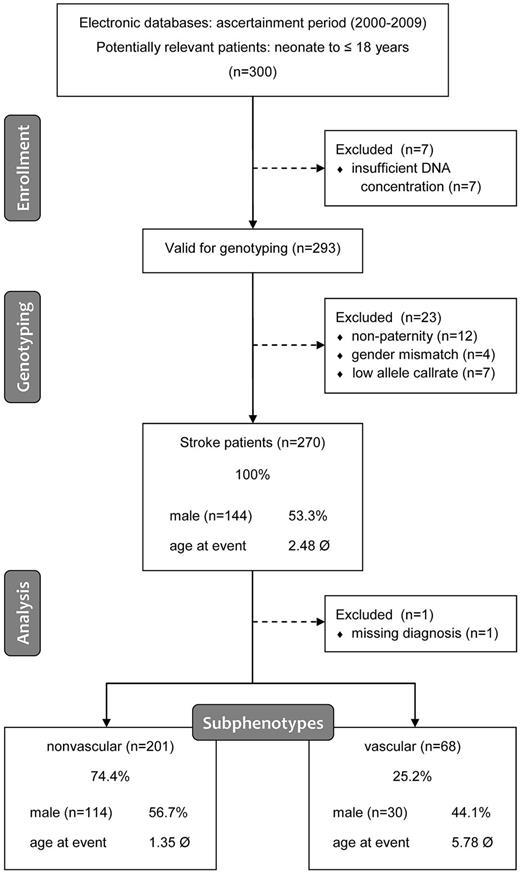
From an initial study sample composing 300 consecutively enrolled families, 293 trios were considered eligible for genotyping after removal of siblings and assessment of DNA quality. Of these, 270 were valid for statistical analysis after filtering for call rate (> 97%), Mendelian inheritance error rate (< 0.1%), and gender prediction match using the GenomeStudio Software (Illumina Version 1.9.4; Figure 1). Potential population stratification was assessed using a population concordance analysis implemented in PLINK Version 1.06 (supplemental Methods).7
Outline of the study cohort. Numbers relate to cases or trios, respectively. In addition, baseline phenotype statistics are given for all groups that were analyzed.
Outline of the study cohort. Numbers relate to cases or trios, respectively. In addition, baseline phenotype statistics are given for all groups that were analyzed.
Genotyping
In the initial analysis, 235 trios were genotyped on Illumina Infinum 370CNV arrays. A total of 35 additional trios were genotyped using its successor, the Infinium 660W chip because of discontinuation of the 370CNV series. The SNP content overlap between both chip layouts is 89.7%. For statistical analysis, we used all SNPs from the 370CNV chip, accepting a slightly reduced sample size for 36.189 SNPs not present on the 660W chip. This does not pose a statistical bias because the number of observations varies for the transmission disequilibrium test dependent on allele frequency and number of heterozygous parents. We tested the validity of integrating genotypes from these chips by running 40 samples on both chips. Allele callings match at 99.48% for common SNPs; thus, we conclude that genotypes can be combined. All genotyped samples were assessed for sufficient call rate, gender estimation match, mendelian error fraction, and population structure before statistical analysis as detailed in supplemental Methods and supplemental Figure 4.
Association analysis
We performed a GWAS using a threshold of P < 1 × 10−5 for suggestive genome-wide association. P values were computed using the transmission disequilibrium test and validated by 20 000 gene-dropping permutation runs yielding empirical P values based on unbiased null distributions (PLINK Version 1.06).7 This analysis was carried out for the complete dataset (n = 270) and a subset excluding vascular strokes (n = 201).
Systems biology analysis
In a subsequent step, we estimated the amount of true association signals by visual inspection of the QQ-plot. Deviating intervals in the QQ-plot are considered as indicators for population stratification or similar statistical bias.8 However, the observed deviation at midrange P values may also be attributed to the presence of a large number of independent and weakly associated loci that reflect the genetic complexity of the disease. As family studies are immune to population stratification and persons were ascertained consecutively, selection bias toward secondary phenotypes is unlikely in our experimental setting.
Therefore, we assessed the excess of observed association in an enrichment analysis for abundant stroke-related genes occurring in deviating P value intervals in the QQ-plot. We assembled a set of stroke-related genes by collecting all gene identifiers that occur with the key word “stroke” in PubMed abstracts (supplemental Methods and supplemental Figure 1). A second set of GWAS-derived genes was generated by setting a threshold at P < 1 × 10−3 in our dataset to generate a set of candidate SNPs, expecting a fraction of 16% among the loci to be true-positive associations according to a false discovery rate of 84% derived by Benjamini-Hochberg correction. These candidate SNPs were then assigned to one or more genes using a linkage disequilibrium (LD) mapping approach and the post-GWAS R package as described in the supplemental data. Over-representation of stroke-associated genes within the GWAS set was determined by Fisher exact test using R (supplemental Figure 2). To account for potential publication bias in this procedure, we performed a permutation test with 100 000 random samples matching the size of the stroke-related gene set, which were drawn from the entire human genome (Ensembl database, Homo sapiens genes GRCh37.p5). The overlap with the GWAS-derived set of genes was computed and results in an approximate null distribution that is used for comparison to the observed overlap for the stroke-related set (supplemental Figure 1).
Second, we investigated the data regarding their relatedness within protein interaction networks to identify low-risk variants that act jointly on an intermediate phenotype (ie, biologic pathway). We used dmGWAS,6 a method that searches for densely connected modules within protein-interaction graphs, where members of such modules are constrained toward an accumulation of strong association signals. In a primary step, a representative P value was assigned to each gene, which is the smallest of (1) all intragenic SNPs and (2) SNPs with an LD r2 > 0.3. Afterward, the dense module search of dmGWAS was performed with an interaction dataset and parameter settings as described in supplemental materials. From the resulting set of modules, we extracted the top 3 for visualization. In addition, we used Gene Ontology and pathway data from Reactome as established categorization databases in an enrichment analysis to identify primary biologic functions of the resulting network. We identified enriched terms in the network of proteins using the over-representation analysis features of the STRING database web interface.9 Proteins containing these terms are colorized accordingly in the graphical plot.
Results
Genome-wide association analysis
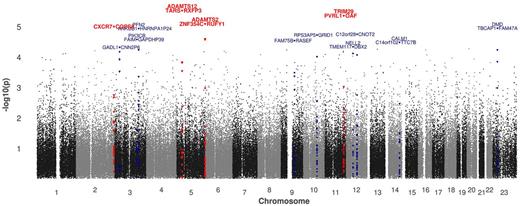
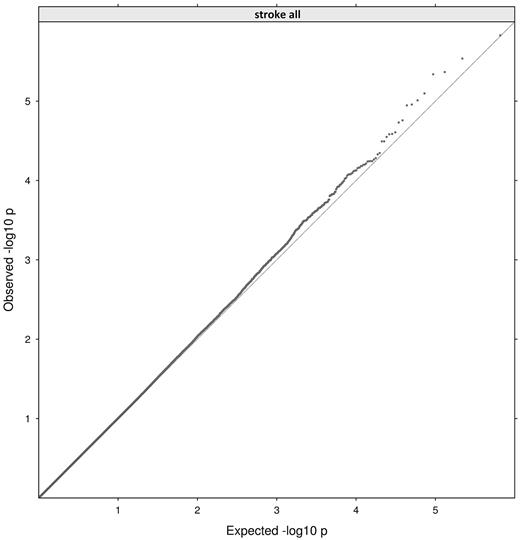
GWAS was conducted in the complete dataset of 270 incident stroke families and a subset of nonvascular stroke (n = 201). A total of 334 581 SNPs provided valid association signals after applying missingness tests (> 25% genotyped) and allele frequency filtering (> 1%), resulting in a signal intensity plot as shown in Figure 2. The accompanying QQ-plot (Figure 3) confirms the presence of causative loci by showing a deviation between the numbers of SNPs at high P values compared with those expected under a null model. In total, 4 hits exceeded the threshold of P < 1 × 10−5 for suggestive genome-wide significance (rs1364044, P = 2.9 × 10−6; rs2084898, P = 4.3 × 10−6; rs469568, P = 8.0 × 10−6; rs4663691, P = 9.8 × 10−6), which are summarized in Table 1. Three SNPs are intragenic; 2 of them are located in genes that belong to the ADAMTS family, one is proximate to COPS8. As shown in Table 1, all loci are robust to permutation testing and range at medium to high allele frequencies (12%-48%) in the population resulting in a large number of heterozygous parents and reliable transmitted/untransmitted minor allele ratios. Comparative display of subphenotype P values and LD information is further shown in regional plots (supplemental Figure 2). Because of the smaller subphenotype datasets, P values drop approximately 1 order of magnitude but do not show major discrepancies because of subphenotype stratification (Table 1).
Manhattan plot showing the distribution of P values over all chromosomes. Loci are colored in correspondence to P value thresholds: red represents P < 10−5; and blue, P < 5 × 10−4. Annotation shows the closest gene in each direction (upstream/downstream, intragenic).
Manhattan plot showing the distribution of P values over all chromosomes. Loci are colored in correspondence to P value thresholds: red represents P < 10−5; and blue, P < 5 × 10−4. Annotation shows the closest gene in each direction (upstream/downstream, intragenic).
Quantile plot comparing observed distribution of P values from our study with the expected distribution of P values under a null model.
Quantile plot comparing observed distribution of P values from our study with the expected distribution of P values under a null model.
Systems biology analysis
In the QQ-plot (Figure 3), we identified an interval between 10−3 < P < 10−5 showing an excess of observed loci beside the peak signal range. In absence of confounding effects, this suggests the presence of true association. To confirm that this midrange deviation is caused by association with the stroke phenotype, we performed an enrichment analysis with stroke-related genes as described in supplemental methods. We observed that 15.7% of moderately associated loci from our study occur in the set of stroke-related genes (1.8% vice versa), which is more than expected and reaches the threshold of significance as assessed by Fisher exact test (P = .019). Additional permutation testing confirmed the findings at P < .0005 (supplemental Methods and supplemental Figure 1).
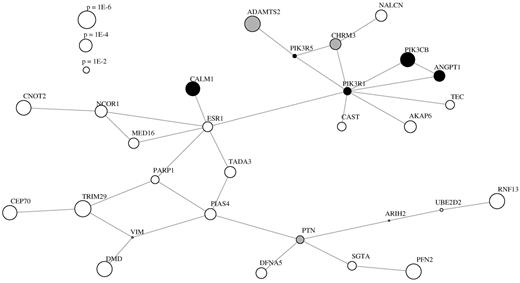
Subsequently, we assessed the entire GWAS dataset for dysregulated molecular networks arising from a cumulative effect of loci with moderate P values. The results of the corresponding dmGWAS analysis are given in Figure 4. We identified 3 different domains of biologic function in this network. The first is composed of genes of transcriptional control (CNOT2, NCOR1, and TRIM29) and cell division (CEP70) without known functional implication in stroke. More interestingly, ADAMTS2 and CHRM3 appear as members of the extracellular matrix and endothelium in a submodule that is tightly coupled to members of the phosphoinositide and calcium signaling pathways (CALM1, PIK3CB). Together with ANGPT1, a carrier of several fibrinogen domains, these are essential mediators of platelet activation. Profilin-2 (PFN2) occupies another central role in this interface between coagulation signaling and extracellular matrix, although appearing in an isolated position in the network. It is an actin-polymerizing protein found in the plasma membrane of platelets that depends on the activity of phosphoinositide.10 An assessment of enriched Gene Ontology biologic processes in the network confirms the central role of blood coagulation with a P value of 9.5 × 10−5 but slightly misses significance after correction for multiple testing using the false discovery rate (P = .055). Further classes of Gene Ontology terms are metabolic processes (P = 2.2 × 10−6, corrected P = .01) and transcription (P = 8.6 × 10−5, corrected P = .055) as listed in supplemental Table 1. DMD as a prominent cardiovascular risk gene is not addressed here because it spans a large genomic region and resides on the X-chromosome and is thus likely a false positive association, which requires careful interpretation.
Analysis of associated loci accumulated in protein interaction network modules as computed by dmGWAS. Proteins that belong to a Reactome pathway containing the string “platelet” have been marked black and gray for “endothel” and “extracellular” accordingly.
Analysis of associated loci accumulated in protein interaction network modules as computed by dmGWAS. Proteins that belong to a Reactome pathway containing the string “platelet” have been marked black and gray for “endothel” and “extracellular” accordingly.
Overlap with GWAS signals previously identified for adult stroke
To investigate whether some genetic components overlap between phenotype windows, we searched for loci that were previously reported to be associated with adult stroke in the literature. We extracted 710 SNP loci as putative candidates and mapped them to our dataset using a proxy SNP approach (supplemental Methods). However, none of these loci reached significance after correction for multiple testing.
Discussion
Here we present results of the first GWAS on pediatric stroke in 270 nuclear families. To our knowledge, this cohort represents the largest study sample currently available worldwide. We identified several novel susceptibility genes that directly relate to coagulation and vascular biology. The most important finding is the identification of 4 members of the ADAMTS gene family at confident association levels. Although SNPs residing in ADAMTS2 and ADAMTS12 are strongly associated (P = 8.0 × 10−6, P = 2.9 × 10−6), SNPs in ADAMTS13 and ADAMTS17 showed moderate association (P = 9.3 × 10−4, P = 8.5 × 10−4) with pediatric stroke. ADAMTS13, also known as VWF cleaving protease, is already established as a crucial factor in blood coagulation and stroke,11 reduces platelet adhesion and aggregation,12 and down-regulates thrombus formation and inflammation.13 Reduction or absence of ADAMTS13 activity results in impaired degradation of ultra-large VWF multimers and leads to excessive VWF-induced platelet aggregation on the endothelium. Mutations in the ADAMTS13 gene have been shown to cause familial thrombotic thrombocytopenic purpura, which is associated with brain ischemia and neurologic symptoms because of microthrombi in the microvasculature.14 These observations are further supported by an experimental study, where ADAMTS13 gene deletion aggravated ischemic brain damage in a reperfusion mouse model.15
In the clinical setting, ADAMTS13 has been proposed as a target for treatment of stroke because of its negative regulation of thrombosis and inflammation.16 In addition, acquired thrombotic thrombocytopenic purpura has been described in the presence of IgG, IgA, or IgM antibodies inhibiting ADAMTS13 activity. In these cases, the inhibitory antibody effect could be overridden by administration of recombinant ADAMTS13 concentrate.17 In an experimental study, Zhao et al reported that infusion of high dose ADAMTS13 concentrates administered immediately before reperfusion injury significantly reduces infarct volume in mice and improves functional outcome without evidence of hemorrhage.16 Taken together, these studies render ADAMTS13 an attractive candidate for clinical treatment of stroke and a potential diagnostic kit that incorporates genetic variation as predictors for stroke risk.
Several other genes from the ADAMTS family, such as ADAMTS7, have already been connected to endothelial differentiation and matrix physiology.18 Recent meta- analyses revealed an association of ADAMTS7 and ADAMST8 with pulse pressure19 and coronary artery disease20 at genome-wide significance. Notably, ADAMTS7 is a direct phylogenetic neighbor of ADAMTS12 (supplemental Figure 3), which is the gene with the most stringent association in our study. The large amount of sequence identity between the 2 genes is a strong indicator of redundant functionality. ADAMTS18 has been shown to be protective against stroke in mice as it is capable of dissolving platelet aggregates.21 Thus, a general role of the ADAMTS gene family in extracellular matrix biology and coagulation is conceivable based on our data and existing knowledge. Nevertheless, for ADAMTS2 and ADAMTS12, the susceptibility genes with most significant association signals, there is no information on biologic function available yet. ADAMTS2 is known to be involved in procollagen processing, inhibition of angiogenesis, and tumor growth.22 ADAMTS12 shows similar functionality, being involved in the degradation of cartilage oligomeric matrix protein and showing antiangiogenic and tumor-suppressive properties23 as well as a modest association with heart failure.24 However, the mechanisms through which the genetic variants in ADAMTS genes lead to pediatric stroke remain unknown.
We observed further loci at suggestive genome-wide significance that do not map unambiguously to a distinct gene. One variant tags a LD block containing the genes TRIM29, OAF, and POU2F3, and the other variant resides in an intergenic region proximal to COPS8. The TRIM29 block is a cassette of genes involved in regulation of transcription. COPS8 (as part of the protein complex COP9) is a regulator of cyclooxogenase-225 and might therefore influence coagulation. It has been argued that variants identified in GWAS explain only a small proportion of the genetic variance underlying most complex traits.5
Particularly for a phenotype, such as stroke, the physiologic heterogeneity can manifest as locus heterogeneity, where a larger number of loci with small effects affects relevant pathways in a cumulative fashion. The severity of pediatric diseases with an early onset hints toward the presence of individual rare mutations scattered over a larger number of causal genes, resulting in poor association strength.26 Therefore, we applied dmGWAS to identify densely connected modules in the human protein interactome that conjoins loci of strong to moderate significance. This network provides first insights into the interplay of putative causative genes on a proteome level and represents a first attempt to understand gene variants in the context of whole cells and cellular communication. Accordingly, we found interacting components of extracellular matrix biology (ADAMTS2, CHRM3, PFN2) and coagulation signaling (CALM1, PIK3CB, PFN2) that suggest a complex relation between multiple causative genes with low to medium individual effects. Significance of these genes is further substantiated by the literature. For PIK3CB, crucial function in platelet aggregation and adhesion to fibrinogen have been shown with targeted alleles in mice.27 Available antithrombotic therapy targeting PI3K28 could be established in the future as a treatment conditional on genetic testing, given successful replication of our results. For ANGPT1, lethal vascular defects have been observed in mouse knockout models, and a special role in injured vasculature has been proposed.29 Interestingly, there is an additional peak at P = 2.0 × 10−4 with the next gene, ANGPT2, residing 380 kb apart, which was not included in the dmGWAS analysis because of our restrictive SNP to gene mapping. CHRM3, also known as muscarinic receptor 3, plays a major role in dilation of coronary arteries on stimulation by acetylcholine in mice.30
Finally, CALM1 has been shown to be significant in an association study for adult stroke31 and is known to be an essential trigger of platelet activation. The abundance of potential risk factors supports a model of heterogeneity as outlined earlier in the “Discussion.” To our knowledge, there is no comparable cohort of pediatric stroke with suitable DNA samples available, mainly because of the low incidence of the disease and restrictions in several countries with such cohorts to conduct genetic association studies in children.
In lack of such an independent study sample, we were not able to validate the association by replication analysis at this time point. And we are aware of the fact that the lack of replication limits our ability to generalize our findings to other cohorts.
In conclusion, this GWAS provides important insights into the genetic underpinnings of stroke in children. We identified a gene family with several members exhibiting considerably strong association signals in the dataset. The observation of 2 genes from the same family within the peak signal range is extraordinary and a novel observation in GWAS and suggests a crucial role for the class of ADAMTS metalloproteinases in pediatric stroke pathogenesis. Second, we have found a cluster of genetic variants that act on common physiologic mechanisms leading to hyper-reactive platelet activation and altered vascular function, and are thus conceivable to cause prothrombotic susceptibility in a cumulative fashion. In conjunction, these results give rise to the hypothesis that a disposition toward a reduced integrity of the endothelial wall as, for example, conferred by ADAMTS defects together with hypersensitive platelet activation and/or vasoconstriction is a major cause of stroke in early childhood. Although our cohort is comparable to similar studies in white children,1,3 the generalizability of our findings hinges on future studies using larger study samples with joint analyses of susceptibility variants. Such studies would also enable the inclusion of intermediate phenotypes and rare genetic variants (identified through next generation sequencing efforts currently underway) to unravel genetic susceptibility and underlying biologic pathways.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all participants from the ESPED registry (English translation of Erhebungseinheit für seltene pädiatrische Erkrankungen in Deutschland).
This work was supported by the Förderverein “Schlaganfall und Thrombosen im Kindesalter e.V.” and Interdisziplinäres Zentrum für Klinische Forschung (CRA01-09), University of Münster.
Authorship
Contribution: M.S. and U.N.-G. conceived and designed the study; U.N.-G., A.A., and M.H. acquired data; M.H. performed the statistical analysis; G.K., K.K., D.M., and U.N.-G. recruited patients; A.A., M.H., U.N.-G., and M.S. drafted the report; A.W. performed all genome-wide genotypings on the core-facility of the Leibniz-Institute for Arteriosclerosis Research; and all authors contributed to the interpretation of data and critically revised the report for important intellectual content.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Monika Stoll, Genetic Epidemiology of Vascular Disorders, Leibniz-Institute for Arteriosclerosis Research, Westfälische Wilhelms University of Muenster, Albert-Schweitzer-Campus 1, D3, 48149 Muenster, Germany; e-mail: mstoll@uni-muenster.de; and Ulrike Nowak-Göttl, Thrombosis & Hemostasis Treatment Center, Institute of Clinical Chemistry, University Hospital Schleswig-Holstein, Campus Kiel, Arnold-Heller-Strasse 3, Building 17, 24105 Kiel, Germany; e-mail: leagottl@uksh.de.
References
Author notes
A.A. and M.H. contributed equally to this study.
M.S. and U.N.-G. are co–senior authors.