In this issue of Blood, Weller et al studied patients with rare immunodeficiencies to demonstrate a role for Toll-like receptors (TLRs) in homeostasis of human CD27+IgM+IgD+ B cells.1
Human CD27+IgM+IgD+ B cells are a peculiar subset, the origin and function of which are debated. These cells display typical characteristics of memory B cells: a rapid in vitro response to activation signals, up-regulation of activation markers, somatic hypermutations in their immunoglobulin (Ig) genes, and extensive replication history.2,3 However, CD27+IgM+IgD+ B cells phenotypically resemble splenic marginal zone B cells that are presumed to respond independent of T-cell help.4 Still, circulating CD27+IgM+IgD+ B cells are 2- to 3-fold reduced in CD40L-deficient patients,3 and cells within this population display molecular signs of a germinal center origin.5 Thus, in healthy adults, CD27+IgM+IgD+ B cells likely constitute a mixture of germinal center and marginal zone-derived memory B cells (see figure panel A).3
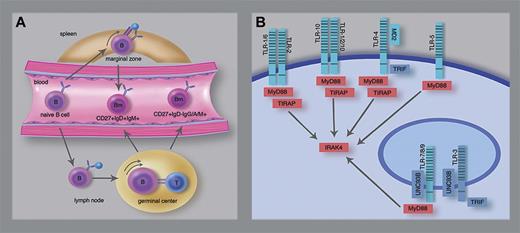
TLR signaling in human CD27+IgM+IgD+ B cells. (A) Differentiation scheme of CD27+IgM+IgD+ and CD27+IgD- memory B cells. Human CD27+IgM+IgD+ B cells in the splenic marginal zone are thought to derive from T cell–independent responses and able to circulate in peripheral blood. CD27+IgD-IgM+, IgA+ or IgG+ memory B cells and at least part of the CD27+IgM+IgD+ B cells derived from T cell–dependent germinal center responses. (B) Scheme of human TLRs and critical signaling molecules. Weller et al demonstrated that the presumed marginal zone-derived CD27+IgM+IgD+ B cells depend on MyD88, TIRAP, and IRAK4 (red), but not on TLR3, UNC93B, or TRIF (blue). TLRs form homodimers, except for TLR1 and TLR6 which function as heterodimers with TLR2. TLR10 potentially functions as homodimer or as heterodimer with TLR1 or TLR2. Professional illustration by Marie Dauenheimer.
TLR signaling in human CD27+IgM+IgD+ B cells. (A) Differentiation scheme of CD27+IgM+IgD+ and CD27+IgD- memory B cells. Human CD27+IgM+IgD+ B cells in the splenic marginal zone are thought to derive from T cell–independent responses and able to circulate in peripheral blood. CD27+IgD-IgM+, IgA+ or IgG+ memory B cells and at least part of the CD27+IgM+IgD+ B cells derived from T cell–dependent germinal center responses. (B) Scheme of human TLRs and critical signaling molecules. Weller et al demonstrated that the presumed marginal zone-derived CD27+IgM+IgD+ B cells depend on MyD88, TIRAP, and IRAK4 (red), but not on TLR3, UNC93B, or TRIF (blue). TLRs form homodimers, except for TLR1 and TLR6 which function as heterodimers with TLR2. TLR10 potentially functions as homodimer or as heterodimer with TLR1 or TLR2. Professional illustration by Marie Dauenheimer.
The redundancy of CD40-dependent signaling in the generation of marginal zone–like CD27+IgM+IgD+ B cells implies a role for other stimuli, for example, through pattern recognition receptors of the Toll family (TLR). Humans have 10 TLRs, of which TLR9 and TLR10 transcripts are highly expressed and TLR1, TLR6, and TLR7 weakly expressed in B cells.2 To assess the in vivo role of TLR signaling in the homeostasis of circulating marginal zone-like B cells, Weller et al studied presence of CD27+IgM+IgD+ versus CD27+IgD- memory B cells in a unique series of patients with rare genetic defects.1 Deficiencies of UNC93B, TRIF, or TLR3 did not impair circulating CD27+IgM+IgD+ nor CD27+IgD- B cells indicating that TLR3, TLR7, TLR8, and TLR9 are dispensable for homeostasis of B-cell memory (see figure panel B). In contrast, MyD88, IRAK4, and TIRAP deficiencies specifically affected CD27+IgM+IgD+, but not CD27+IgD- memory B cells. Because TLR4 and TLR5 are not expressed in human B cells, only TLR1, TLR2, TLR6, and TLR10 remain potential candidates for a nonredundant function in marginal zone–like B cells. Of these, TLR2 forms heterodimers with either TLR1 or TLR6 for recognition of triacyl and diacyl lipopeptides, respectively. TLR10 is thought to recognize lipoteichoic acid, either as a homodimer or a heterodimer with TLR1 or TLR2.6 The high expression levels of TLR10 make this a more interesting candidate receptor.1,2 However, the function of TLR10 has not been well established, because it is: (1) not present in the mouse, and (2) fails to activate typical TLR target genes.6
Insights into human T cell–dependent and –independent B-cell responses cannot be straightforwardly translated from mouse models. Mice have a different anatomical make up of their splenic marginal zone and they carry few memory B cells with mutated Ig genes, probably due to their specific-pathogen-free housing. The discovery of various new genetic defects in patients with primary immunodeficiencies has greatly enhanced possibilities to perform in vivo functional analysis of the human immune system. By showing that CD27+IgM+IgD+, but not CD27+IgD- memory B cells depend on MyD88-IRAK4-TIRAP signaling, Weller et al identified the first pathway that is specifically required for these cells.1 Moreover, their results support the concept that pattern recognition receptors can signal for maturation in B cells that are not dependent on cognate T-cell help. Because (for obvious reasons) the authors were unable to directly study patients' spleens, it remains unclear whether the defect in circulating CD27+IgM+IgD+ B cells is the result of impaired responses in the marginal zone. Interestingly, these cells are markedly reduced, but not completely absent, in both CD40- and in MyD88/TIRAP/IRAK4-signaling deficient patients.1,3 This could imply that both pathways are required for their homeostatic maintenance, or that in healthy individuals CD27+IgM+IgD+ B cells are a mixture of 2 subsets produced by 2 distinct pathways. In case of the latter, it can be anticipated that T-cell independently–derived memory B cells will display fewer somatic hypermutations and in vivo proliferation.3 Comparative phenotyping or transcription profiling of CD27+IgM+IgD+ B cells in CD40L and MyD88/TIRAP/IRAK4 deficiency could facilitate the identification of novel markers to discriminate these pathways in healthy individuals.
In addition to marginal zone responses, the approach of Weller et al can be applied to study TLR signaling in other T cell–independent responses. Recently, it was shown that CD27-IgA+ memory B cells are not dependent on CD40 signaling and display a low replication history, high IgA2 subclass and Igλ usage, reminiscent of T cell–independent responses in the human intestinal tract.3 It would be interesting to study whether these cells depend on similar or distinct signaling pathways as CD27+IgM+IgD+ memory B cells.
Splenic marginal zone B cells are implicated in the response against encapsulated bacteria. TIRAP-dependent TLR1, TLR2, and TLR10 recognize structures that are typically present in the bacterial cell wall. MyD88/IRAK4/TIRAP-deficient patients suffer from recurrent bacterial infections early in life, but seem to overcome this when growing older.7 It is currently unclear whether the defect in CD27+IgM+IgD+ B cells contributes to the high frequency of infections, but the reduction of infection with older ages suggest that these patients develop (CD27+IgD-) B-cell memory. Furthermore, recurrent bacterial infections in patients with an antibody deficiency are not reported to result from a specific defect in CD27+IgM+IgD+ B cells. Rather, these patients display defects in both CD27+IgM+IgD+ and CD27+IgD-, or only CD27+IgD- B cells.8 Thus, further studies are required to assess the nonredundant role of CD27+IgM+IgD+ B cells in human immunity.
Besides inherited defects that impair TLR signaling, somatic mutations in MyD88 have recently been identified that constitutively activate MyD88-dependent signaling in lymphomas. Interestingly, these mutations have been identified in marginal zone lymphomas,9 as well as in germinal center B-like diffuse large B-cell lymphomas.10 Constitutive MyD88-dependent signaling in these lymphomas is critical for their survival.10 However, Weller et al here show a differential requirement of CD27+IgM+IgD+ and CD27+IgD- B cells on TLR signaling.1 This concept is important for further studies on T cell–independent B-cell activation, circulating marginal zone–like B cells, and human TLR10 function. Insights into these processes have direct implications for diagnosis and treatment of patients with immunodeficiencies or B-cell malignancies.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■