Abstract
Abstract 601
Despite therapeutic advances and availability of novel agents, multiple myeloma (MM) remains an incurable malignant disorder and strategies to improve survival are being actively sought. One such approach is the use of maintenance therapy with lenalidomide (Revlimid®; R), which has shown PFS benefit, without conclusive evidence of OS benefit except in a selected population (CALGB100104). Shrinking resources with rising healthcare costs warrant careful assessment of the financial impact of expensive anticancer therapeutics in context with the true benefit to patients.
This analysis was not supported or solicited by any pharmaceutical industry. For our pharmacoeconomic study we selected the MM-015 trial; a large (n=459) double-blind, randomized, placebo-controlled phase III study that investigated the efficacy of continuous R maintenance after initial therapy with melphalan/prednisone/lenalidomide (MPR) in a uniform cohort of patients (≥65 years, newly diagnosed MM, transplant ineligible). The three study arms were MP, MPR and MPR followed by R maintenance (MPR-R). The cost-effectiveness model included medical costs (drugs, office visits, laboratory tests) and costs of treatment-related adverse events (AEs) for an individual patient. Drug costs were calculated from the average wholesale price, using average US adult weight of 80kg. Office visit and laboratory test costs were obtained using Medicare reimbursement fees. Costs of AEs were obtained from peer-reviewed publications. Incidence of AEs was obtained from the published MM-015 trial. Costs were valued in 2011 US dollars. Cost calculations included: (1) computing distribution of the numbers of induction and maintenance courses completed by patients treated with each of these regimens; (2) computing the per-course probabilities of the occurrence of individual AEs during induction and maintenance for each of these toxicities; (3) combining these data with the treatment costs and costs of individual AEs to compute cumulative cost of treatment through 40 maintenance cycles and cumulative cost of treatment per progression-free survivor. Summary measures computed for each treatment: (1) average cumulative cost per patient (ACCP) treated; (2) average cumulative cost per progression free survivor (ACCPFS), representing the average cumulated cost/patient who is progression free at that time. The distribution of courses received was obtained from the MM-015 publication. Data on subsequent therapy at the time of disease progression was not available.
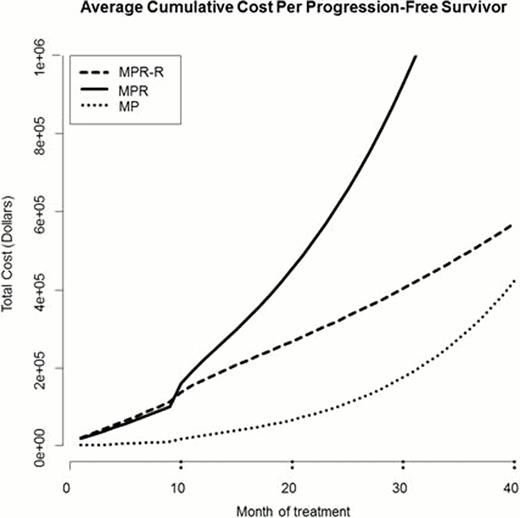
Median follow up reported in the published study was 30 months. Median PFS was 13 months (MP), 14 months (MPR) and 31 months (MPR-R) in the three arms. Table 1 summarizes the calculated ACCP and the ACCPFS of each regimen for induction and maintenance treatment. MPR-R was the most expensive of the three arms in terms of total cumulative costs, with an ACCP of $231,314, compared to $166,211 for MPR and $17,972 for MP. However, taking the efficacy of each regimen into account (ACCPFS), MPR greatly exceeds both MPR-R and MP ($1,540,139 vs. $516,322 vs. $309,346), while the difference between MPR-R and MP was less distinct. Figure 1 illustrates the exponential increase in ACCPFS of MPR, compared to MPR-R and MP.
| Treatment . | . | ACCP . | ACCPFS . | ||||
|---|---|---|---|---|---|---|---|
| . | MP . | MPR . | MPR-R . | MP . | MPR . | MPR-R . | |
| Induction (9 cycles) | Medical | 2,185 | 41,575 | 41,575 | 2,614 | 48,272 | 48,273 |
| AE | 7,885 | 55,081 | 55,081 | 9,431 | 63,954 | 63,956 | |
| Total | 10,071 | 96,656 | 96,656 | 12,045 | 112,226 | 112,229 | |
| Maintenance (36 cycles) | Medical | 3,989 | 71,392 | 139,536 | 68,660 | 661,532 | 311,461 |
| AE | 13,983 | 94,819 | 91,778 | 240,686 | 878,607 | 204,861 | |
| Total | 17,972 | 166,211 | 231,314 | 309,346 | 1,540,139 | 516,322 | |
| Treatment . | . | ACCP . | ACCPFS . | ||||
|---|---|---|---|---|---|---|---|
| . | MP . | MPR . | MPR-R . | MP . | MPR . | MPR-R . | |
| Induction (9 cycles) | Medical | 2,185 | 41,575 | 41,575 | 2,614 | 48,272 | 48,273 |
| AE | 7,885 | 55,081 | 55,081 | 9,431 | 63,954 | 63,956 | |
| Total | 10,071 | 96,656 | 96,656 | 12,045 | 112,226 | 112,229 | |
| Maintenance (36 cycles) | Medical | 3,989 | 71,392 | 139,536 | 68,660 | 661,532 | 311,461 |
| AE | 13,983 | 94,819 | 91,778 | 240,686 | 878,607 | 204,861 | |
| Total | 17,972 | 166,211 | 231,314 | 309,346 | 1,540,139 | 516,322 | |
From a cost-effectiveness perspective, if MPR is used as the induction regimen, maintenance with R should be strongly considered, as MPR without maintenance does not confer any significant benefit over MP, while incurring a much higher cost. However, the overall cost of MPR-R is substantial with no reported OS benefit at present. In the absence of clear survival benefit the financial burden of prolonged treatment becomes an important factor to consider prior to adopting this therapeutic strategy in MM. As part of a global strategy for addressing healthcare costs, it is important to consider this in appropriate medical decision making for patients, and to include cost-effectiveness analyses as a part of large prospective clinical trial efforts.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.