Abstract
Abstract  599
599
This study was undertaken to develop selection guidelines for transplant centers to determine eligibility for stem cell transplantation in patients with light chain amyloidosis.
Patients with biopsy-confirmed immunoglobulin light chain amyloidosis who underwent stem cell transplantation between March 8, 1996, and December 31, 2011, were reviewed in 2 cohorts: those who underwent transplantation between March 8, 1996, and June 30, 2009, and those who underwent transplantation between July 1, 2009, and December 31, 2011 (table 1). A second comparison was undertaken among patients who died before posttransplant day 100 to determine features predictive of early death (table 2).
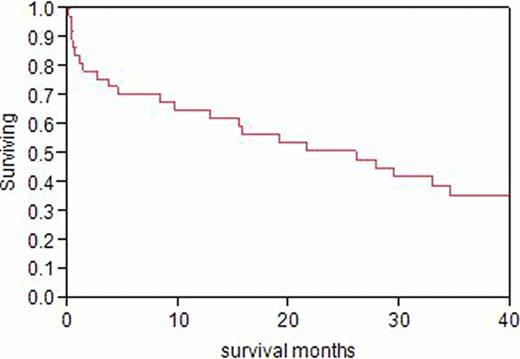
A total of 499 patients were identified, 410 in the earlier group and 89 in the later group. After July 1, 2009, significantly fewer transplant recipients had Mayo stage III cardiac involvement. Mortality before posttransplant day 100 was 10.5% (43/410) in the earlier group and 1.1% (1/89) in the later group. In the earlier group, one-quarter of transplant recipients with N-terminal pro-brain natriuretic peptide (NT-proBNP) levels higher than 5,000 pg/mL died by 10.3 months (fig 1, left). When the serum troponin T level was > 0.06 ng/mL, 25% died at 3.7 months (fig 2, right).
The Mayo staging system is highly predictive for overall survival but not useful for selecting transplant recipients. Patients with serum troponin T levels higher than 0.06 ng/mL or NT-proBNP levels >5,000 pg/mL (not on dialysis) should not be considered acceptable candidates for stem cell transplantation because of unacceptable early mortality.
Comparison of Patients Receiving Transplants Before and After July 1, 2009
| Characteristic Compared . | Transplant Before 7/1/09 (n=410) . | Transplant After 7/1/09 (n=89) . | P Value . |
|---|---|---|---|
| Mayo stage, No. (%)a | .02 | ||
| I | 104/281 (37) | 44/85 (52) | |
| II | 115/281 (41) | 31/85 (36) | |
| III | 62/281 (22) | 10/85 (12) | |
| Male, No. (%) | 245 (60) | 54 (61) | .78 |
| Heart involved, No. (%) | 208 (51) | 31 (35) | .006 |
| Kidney involved, No. (%) | 285 (70) | 61 (69) | .90 |
| Albumin, median (IQR), g/dL | 2.7 (1.9–3.3) | 2.6 (2.1–3.3) | .43 |
| Creatinine, median (IQR, mg/dL) | 1.8 (0.9–1.3) | 1.0 (0.8–1.2) | .002 |
| (10%>1.8) | (10%>1.5) | ||
| Marrow PC, median (IQR), % | 7 (3–13) | 8.5 (4–14) | .04 |
| Septal thickness, median (IQR), mm | 12 (10–14) | 11 (10–14) | .08 |
| EF, median (IQR), % | 65 (60–69) | 64 (61–67) | .31 |
| Age, median (IQR), y | 57.5 (51–63) | 58.4 (54–63) | .18 |
| Troponin T, median (IQR), ng/mL | 0.01 (0.01–0.03) | 0.01 (0.01–0.02) | .1 |
| iFLC, median (IQR), mg/dL | 15.5 (7.3–37.4) | 11.4 (5.8–29.8) | .05 |
| NT-proBNP, median (IQR), pg/mL | 662 (166–2,766) | 322 (125–1,100) | .02 |
| (10%>6,537) | (10%>4,023) |
| Characteristic Compared . | Transplant Before 7/1/09 (n=410) . | Transplant After 7/1/09 (n=89) . | P Value . |
|---|---|---|---|
| Mayo stage, No. (%)a | .02 | ||
| I | 104/281 (37) | 44/85 (52) | |
| II | 115/281 (41) | 31/85 (36) | |
| III | 62/281 (22) | 10/85 (12) | |
| Male, No. (%) | 245 (60) | 54 (61) | .78 |
| Heart involved, No. (%) | 208 (51) | 31 (35) | .006 |
| Kidney involved, No. (%) | 285 (70) | 61 (69) | .90 |
| Albumin, median (IQR), g/dL | 2.7 (1.9–3.3) | 2.6 (2.1–3.3) | .43 |
| Creatinine, median (IQR, mg/dL) | 1.8 (0.9–1.3) | 1.0 (0.8–1.2) | .002 |
| (10%>1.8) | (10%>1.5) | ||
| Marrow PC, median (IQR), % | 7 (3–13) | 8.5 (4–14) | .04 |
| Septal thickness, median (IQR), mm | 12 (10–14) | 11 (10–14) | .08 |
| EF, median (IQR), % | 65 (60–69) | 64 (61–67) | .31 |
| Age, median (IQR), y | 57.5 (51–63) | 58.4 (54–63) | .18 |
| Troponin T, median (IQR), ng/mL | 0.01 (0.01–0.03) | 0.01 (0.01–0.02) | .1 |
| iFLC, median (IQR), mg/dL | 15.5 (7.3–37.4) | 11.4 (5.8–29.8) | .05 |
| NT-proBNP, median (IQR), pg/mL | 662 (166–2,766) | 322 (125–1,100) | .02 |
| (10%>6,537) | (10%>4,023) |
Survival (Before/After Posttransplant Day 100) in 410 Patients Who Received Transplants Before July 1, 2009
| Characteristic Compared . | Died Before Day 100 (n=43) . | Survived After Day 100 (n=367) . | P Value . |
|---|---|---|---|
| Mayo stage, No. (%)a | .002 | ||
| I | 4/28 (14) | 100/253 (40) | |
| II | 13/28 (46) | 102/253 (40) | |
| III | 11/28 (39) | 51/253 (20) | |
| Kidney involved, No. (%) | 33 (77) | 252 (69) | .30 |
| Heart involved, No. (%) | 32 (74) | 191 (52) | <.001 |
| Albumin, median (IQR), g/dL | 2.3 (1.7–3.0) | 2.7 (2.0–3.3) | .02 |
| Creatinine, median (IQR), mg/dL | 1.2 (1.0–1.7) | 1.1 (0.9–1.3) | .001 |
| Marrow PC, median (IQR), % | 6 (4–12) | 7 (3–13) | .90 |
| Septal thickness, median (IQR), mm | 14 (12–16) | 12 (10–14) | <.001 |
| EF, median (IQR), % | 66 (56–70) | 65 (60–69) | .97 |
| Age, median (IQR), y | 56 (49–65) | 57 (51–62) | .60 |
| Troponin T, median (IQR), ng/mL | 0.03 (0.01–0.08) | 0.01 (0.01–0.06) | <.001 |
| (10%>0.125) | (10%>0.05) | ||
| iFLC, median (IQR), mg/dL | 21.3 (15–43.7) | 15.2 (6.4–36.5) | .004 |
| NT-proBNP, median (IQR), pg/mL | 2,752 (666–4,645) | 600 (170–1,969) | <.001 |
| (10%>10,753) | (10%>5,383) |
| Characteristic Compared . | Died Before Day 100 (n=43) . | Survived After Day 100 (n=367) . | P Value . |
|---|---|---|---|
| Mayo stage, No. (%)a | .002 | ||
| I | 4/28 (14) | 100/253 (40) | |
| II | 13/28 (46) | 102/253 (40) | |
| III | 11/28 (39) | 51/253 (20) | |
| Kidney involved, No. (%) | 33 (77) | 252 (69) | .30 |
| Heart involved, No. (%) | 32 (74) | 191 (52) | <.001 |
| Albumin, median (IQR), g/dL | 2.3 (1.7–3.0) | 2.7 (2.0–3.3) | .02 |
| Creatinine, median (IQR), mg/dL | 1.2 (1.0–1.7) | 1.1 (0.9–1.3) | .001 |
| Marrow PC, median (IQR), % | 6 (4–12) | 7 (3–13) | .90 |
| Septal thickness, median (IQR), mm | 14 (12–16) | 12 (10–14) | <.001 |
| EF, median (IQR), % | 66 (56–70) | 65 (60–69) | .97 |
| Age, median (IQR), y | 56 (49–65) | 57 (51–62) | .60 |
| Troponin T, median (IQR), ng/mL | 0.03 (0.01–0.08) | 0.01 (0.01–0.06) | <.001 |
| (10%>0.125) | (10%>0.05) | ||
| iFLC, median (IQR), mg/dL | 21.3 (15–43.7) | 15.2 (6.4–36.5) | .004 |
| NT-proBNP, median (IQR), pg/mL | 2,752 (666–4,645) | 600 (170–1,969) | <.001 |
| (10%>10,753) | (10%>5,383) |
No relevant conflicts of interest to declare.
Author notes

This icon denotes a clinically relevant abstract