Abstract
Abstract 4905
While understanding of Acute Myelogenous Leukemia (AML) pathogenesis has advanced greatly in recent years, drug discovery and development have added little to therapy. Ceramide and other sphingolipids are of therapeutic interest for many neoplasms (Jiang Y, DiVittore NA, Kaiser JM, et al. Combinatorial therapies improve the therapeutic efficacy of nanoliposomal ceramide for pancreatic cancer. Cancer Biol Ther. Oct 1 2011;12(7):574–585). Cellular ceramide accumulation favors a pro-apoptotic state, while accumulation of sphingosine-1-phosphate promotes survival. We have targeted the ceramide balance of AML cells in vitro with varying concentrations of liposomal formulations of C6 ceramide (Lip-C6), the sphingosine kinase-1 inhibitor safingol (Lip-Saf), and tamoxifen (Lip-Tam) to determine potential synergistic anti-leukemic efficacy. Safingol is a sphingosine kinase inhibitor currently in phase 1 trials. In addition, Tamoxifen can reverse drug resistance of many cancer cell types (Chapman JV, Gouaze-Andersson V, Messner MC, et al. Metabolism of short-chain ceramide by human cancer cells–implications for therapeutic approaches. Biochem Pharmacol. Aug 1 2010;80(3):308–315.). This has been shown to be due to the ability of Tamoxifen to block the activities of glucosylceramide synthase (GCS) and p-glycoprotein (P-GP), which coordinate to detoxify ceramide at the Golgi membrane. In the present study, liposomal drug formulations were chosen to enhance drug delivery and prevent premature drug metabolism.
The cell lines C1498, HL-60, HL-60/VCR, GFPp210, Wehi-3B, K562, U937, and KG-1 were used in this study. Drugs were synthesized into liposomal formulations by the Penn State Hershey Drug Discovery and Delivery Core laboratory. Cellular viability was measured after treatment with Lip-C6, Lip-Saf, Lip-Tam, or a combination for 48 hours. Synergy and dose-response curves were modeled using CalcuSyn software. Apoptosis and cell proliferation were assessed using flow cytometry after treatment of drug for 24 hours at the calculated IC50 from MTS assays. Autophagy was also measured in C1498 cells to confirm an established safingol cell-death mechanism. Primary human AML collected by our lab from consenting, patients was assessed in methylcellulose for blast clonogenicity.
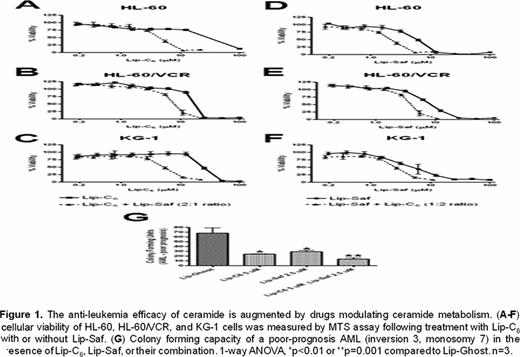
Several cell lines showed a favorable change in the IC50 of the drugs when used in combination, indicating a possible synergistic anti-leukemic mechanism of action. When Lip-C6 was combined with Lip-Saf in a varying ratios, synergistic growth inhibition was observed in the human AML cell lines HL-60, HL-60/VCR, and KG-1 (Figure 1). Interestingly, Lip-Tam caused complete cell population death at concentrations less than 15 μM in the Wehi-3B, K562, GFPp210, and C1498 lines. When cells were treated with Lip-C6 and Lip-Tam in a 1:1 combination, complete cell population killing was noted at concentrations of less than 10 μM in every cell line tested. Additionally, flow cytometric data confirmed findings of other investigators suggesting that Lip-Saf caused enhanced autophagy. Therefore, the observed synergistic leukemia cell death is likely due in part to the novel combination of an autophagy-inducer with an apoptosis-inducer. Clonogenic data has shown that combination of Lip-C6and Lip-Saf cooperate to inhibit formation of blast colonies from human AML, indicating a potential use in lessening leukemia burden (Figure 1).
In conclusion, novel ceramide-centered drug combinations promote improved cell death of leukemia cell lines via accumulation of ceramide and inhibition of ceramide metabolic pathways. This study acts as a persuasive proof-of-concept of the effect of inhibiting a single ceramide degradation pathway within cells. By inhibiting sphingosine kinase-1 or the GCS activity of P-GP in these cell lines, it becomes apparent that the cellular ceramide balance shifts to favor a pro-apoptotic state.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.