Abstract
Abstract 478
Iron is essential for the function of many key proteins including hemoglobin (Hgb) and myoglobin (involved in oxygen transport/exchange), cytochromes (involved in energy generation), various enzymes (involved in cell proliferation and neurotransmission), and immune function. Iron deficiency can, therefore, negatively impact patients' health-related quality of life (HRQL) and requires treatment to replete iron stores. Although oral iron is a common initial treatment, some patients do not tolerate it or fail to adequately respond; many of these patients, therefore, live with chronic anemia and the related negative effects on their overall HRQL. Ferumoxytol (FER) is a new IV iron indicated for the treatment of Iron Deficiency Anemia (IDA) in patients with chronic kidney disease (CKD). FER is being investigated to treat IDA patients without CKD who have a history of unsatisfactory oral iron therapy or in whom oral iron cannot be used. To prospectively explore the impact of FER treatment on patient reported outcomes (PROs), the Phase 3 clinical trials included: the Functional Assessment of Chronic Illness Therapy-Fatigue Scale (FACIT-Fatigue); the Short Form Health Survey (SF-36); and the Linear Analogue Scale Assessment (LASA) of Energy, Activities of Daily Living (ADL) and HRQL.
IDA patients with Hgb <10.0 g/dL and >7 g/dL, and transferrin saturation (TSAT) <20% were eligible. Eight hundred-eight subjects were randomized 3:1 to receive either 2 injections of 510 mg of FER administered 5±3 days apart or IV normal saline (placebo). FACIT-Fatigue assessments were done pre-dose at Baseline and weekly up to Week 5; the SF-36 and LASA were administered at Baseline, Week 3 and Week 5. Comparison of the mean change in FACIT-Fatigue scores from Baseline to Week 5 was a pre-specified secondary endpoint; mean change in the SF-36 Vitality Domain and LASA scores were exploratory endpoints.
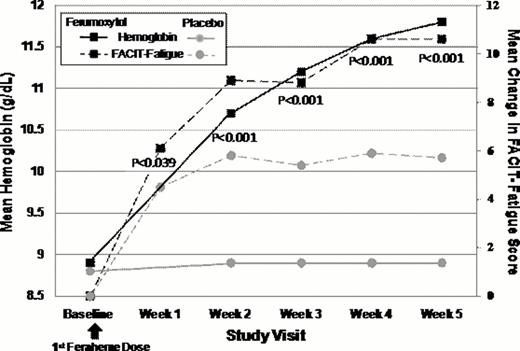
At Baseline, FACIT-Fatigue scores for both treatment groups were lower (FER, 24.0 ±11.8; placebo, 24.7 ±11.3) than general (non-anemic) US population norms (40.1), and comparable to anemic cancer patients receiving chemotherapy (23.9). FER-treated subjects showed a robust 2.7 g/dL increase in mean Hgb from Baseline to Week 5, compared to only a 0.1 g/dL increase with placebo (p<0.0001). Paralleling this Hgb increase, FER-treated subjects also demonstrated large improvements in FACIT-Fatigue scores at each time point with significant improvement as early as Week 1 (+7 point change). Further improvements in fatigue were seen at weeks 2, 3, and 4 after FER treatment, and by Week 5, FACIT-Fatigues scores had increased over 12 points (36.8±11.2) and approached general population norms (40.1). In contrast, FACIT-Fatigue scores in placebo-treated subjects showed a lower increase at Week 1 and no further increase at subsequent time points. At all post-treatment time points, the FACIT-Fatigue score increases in FER-treated subjects were statistically significantly higher than placebo-treated subjects. FER-treated subjects demonstrated significantly greater improvements in SF-36 Vitality scores at each time point following FER treatment relative to placebo. In addition, significantly greater improvements in LASA Energy, ADL and HRQL scores were observed at each time point compared to placebo; these improvements continued to increase for FER at Week 5, but decreased for placebo.
Mean Change in FACIT-Fatigue Scores
| Mean Change* in SF-36 Vitality Scores . | |||
|---|---|---|---|
| Visit . | Ferumoxytol N = 608 . | Placebo N = 200 . | p-value . |
| Week 3 | 9.6 | 5.9 | 0.0002 |
| Week 5 | 10.6 | 5.4 | <0.0001 |
| Mean Change* in LASA Scores | |||
| Energy | |||
| Week 3 | 10.9 | 4.0 | 0.0011 |
| Week 5 | 15.9 | 6.5 | <0.001 |
| Activities of Daily Living | |||
| Week 3 | 7.9 | 2.4 | 0.0106 |
| Week 5 | 11.9 | 3.6 | 0.0003 |
| Quality of Life | |||
| Week 3 | 8.6 | 2.6 | 0.0051 |
| Week 5 | 12.8 | 4.1 | 0.0001 |
| Mean Change* in SF-36 Vitality Scores . | |||
|---|---|---|---|
| Visit . | Ferumoxytol N = 608 . | Placebo N = 200 . | p-value . |
| Week 3 | 9.6 | 5.9 | 0.0002 |
| Week 5 | 10.6 | 5.4 | <0.0001 |
| Mean Change* in LASA Scores | |||
| Energy | |||
| Week 3 | 10.9 | 4.0 | 0.0011 |
| Week 5 | 15.9 | 6.5 | <0.001 |
| Activities of Daily Living | |||
| Week 3 | 7.9 | 2.4 | 0.0106 |
| Week 5 | 11.9 | 3.6 | 0.0003 |
| Quality of Life | |||
| Week 3 | 8.6 | 2.6 | 0.0051 |
| Week 5 | 12.8 | 4.1 | 0.0001 |
Least Square Mean.
These data suggest that FER may provide important clinical benefits to IDA patients with a history of unsatisfactory oral iron therapy; these benefits include reductions in fatigue, increased energy, vitality, and ability to perform ADL and improved HRQL. This is important especially in light of the poor baseline energy and HRQL scores of patients who cannot take oral iron. FER could, therefore, provide an important, new treatment option for IDA patients with a history of unsatisfactory oral iron therapy or in whom oral iron cannot be used.
Vadhan Raj:AMAG Pharmaceuticals, Inc.: Research Funding. Off Label Use: Feraheme (ferumoxytol) injection. For treatment of iron deficiency anemia in non-CKD patients. Hsieh:AMAG Pharmaceuticals, Inc.: Employment. Strauss:AMAG Pharmaceuticals, Inc.: Employment. Stevenson:AMAG Pharmaceuticals, Inc.: Employment. Bernard:AMAG Pharmaceuticals, Inc.: Employment. Allen:AMAG Pharmaceuticals, Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.