Abstract
Abstract 4639
Quantification of immature, i.e. RNA-containing platelets (PLT) was introduced into routine diagnostics by Sysmex as immature platelet fraction (IPF) some years ago and recently by Abbott as reticulated platelets (rPLT). In this study, we evaluated the precision and agreement of both analyzers in normal and pathological samples and correlation of IPF or rPLT with clinical situations.
Anonymized blood samples of cancer patients after chemotherapy, patients with PLT destruction (immune thrombocytopenia, ITP, or heparin-induced thrombocytopenia, HIT), and normal controls were analyzed with double measurements with both, the Abbott CD-Sapphire and Sysmex XE-5000. Furthermore, longitudinal evaluations after chemotherapy were performed and effects of PLT transfusions were analyzed. Precision and agreement were assessed with Bland-Altman analyses and Pearson correlation.
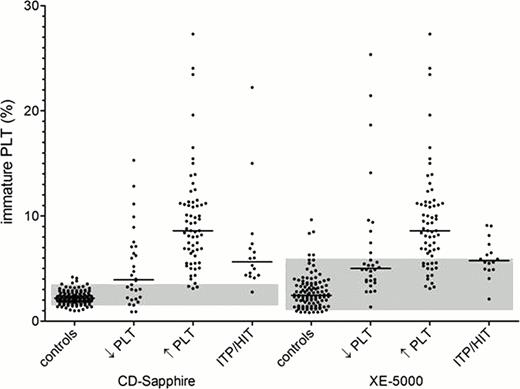
In a first set of experiments, a total of 220 blood samples were analyzed. We studied patients after chemotherapy with beginning thrombocytopenia (30 samples, median PLT count 23/nl) or recovering PLT counts (64 samples, PLT 48/nl), patients with PLT destruction (16 samples, PLT 84/nl) and normal controls (110 samples, PLT 249/nl). Both analyzers demonstrated acceptable overall precision (reproducibility) with lower precision in thrombocytopenia and a non-significant trend towards a better precision with XE-5000. Inter-instrument comparison showed a moderate correlation (Pearson r2=0.38) and a systematic bias of 1.04 towards higher IPF-values with the XE-5000 in the Bland-Altman analysis. Regarding different patient groups, the reference range (percentile 5 – 95) of normal controls was much narrower with the CD-Sapphire, leading to a clear separation of patients with recovering platelet counts after chemotherapy and IPT/HIT, both with increased rPLT as expected (figure). The same trend was visible for the XE-5000, however, with a much broader overlap to the normal range. In longitudinal studies, increased IPF or rPLT were frequently observed already during early thrombocytopenia with a decrease following platelet transfusions.
Correlation between both analyzers for measurement of IPF and rPLT, respectively, was moderate. Precision of measurements was low in thrombocytopenic samples. Samples with abnormally increased IPF/rPLT could be indentified with both analyzers, however, separation from normal was better with the CD-Sapphire. Prediction of PLT recovery after intensive chemotherapy by the measurement of IPF/rPLT is questionable.
Immature PLT counts in patients with beginning thrombocytopenia (↓PLT) or recovering PLT counts (↑PLT) after chemotherapy, PLT destruction (ITP/HIT) and normal controls with the Abbott CD-Sapphire and Sysmex XE-5000. Reference range (percentile 5 – 95) = grey rectangle.
Immature PLT counts in patients with beginning thrombocytopenia (↓PLT) or recovering PLT counts (↑PLT) after chemotherapy, PLT destruction (ITP/HIT) and normal controls with the Abbott CD-Sapphire and Sysmex XE-5000. Reference range (percentile 5 – 95) = grey rectangle.
Krause:Beckman Coulter: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.