Abstract
Abstract 4387
A single-chain variable antibody fragment (scFv) is an engineered protein corresponding to the antibody's antigen-recognizing (variable) domains (VH and VL), connected with an artificial linker. Due to the inherited specificity to the respective antigen, scFv are useful in a broad range of applications. A relatively small size (∼30 kDa) of scFv makes them easy to produce using the recombinant DNA technology. A particular scFv, known as KM33, was previously derived from a patient with anti-factor VIII (FVIII) auto-antibodies manifested in a functional deficiency of FVIII (inhibitory Hemophilia A). KM33 was described as a high affinity ligand of FVIII inhibiting its major functions: procoagulant activity and interactions with von Willebrand factor, phospholipids and catabolic receptors (van den Brink E. et al, 2001 and Limburg V. et al, 2005).
Our goal was to develop a methodology for fast and easy production of KM33 and test its applicability for selective inhibition of FVIII in functional assays.
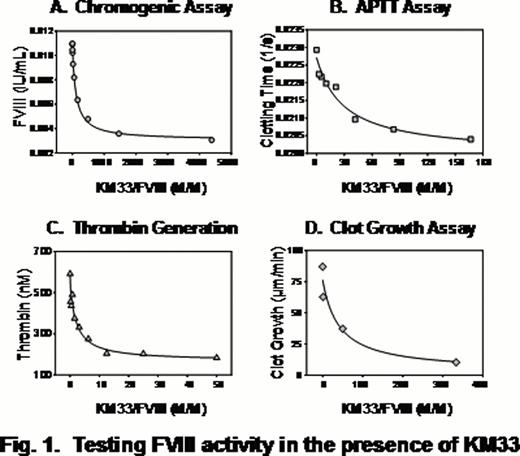
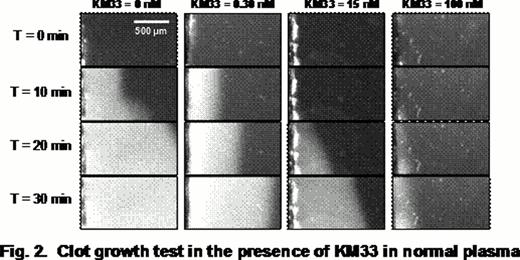
As an alternative to a previously described expression of KM33 in bacteria, we used insect cells capable to perform majority of posttranslational modifications and produce correctly folded proteins. Previously published KM33 coding sequence (Mertens K. et al, 2008) was codon-optimized to the insect host cells (Spodoptera frugiperda), followed by the gene's chemical synthesis, cloning and expression in a baculovirus system. A secretable form of KM33 was purified from the culture media using nickel-affinity and size-exclusion chromatography. The structure and properties of the expressed protein were tested by binding to FVIII in a surface plasmon resonance assay and by measuring its effect on FVIII activity in a chromogenic substrate (CS) assay, clotting assay (APTT), thrombin-generation test (TG) and video microscopy of clot growth (CG).
The insect-cell derived KM33 demonstrated high affinity to FVIII (KD 0.1–0.2 nM) and strong inhibitory effect on the FVIII activity in all assays. At the same time, the inhibition efficiency, assessed by KM33/FVIII molar ratio corresponding to 50% of the inhibitory magnitude, was different between assays. The strongest inhibition was observed in the TG assay, and the weakest in the APTT assay (Fig. 1 and 2). Furthermore, the effect reached an apparent saturation: about 5%, 28% and 42% of FVIII activity remained uninhibited in the TG, CS and APTT assays, respectively. Control experiments performed at highest concentrations of KM33 in the absence of FVIII showed absence of non-specific effects of KM33.
Altogether, our data shows highly specific interaction of KM33 and FVIII including the complex milieu of plasma, and applicability of KM33 for the inhibition of FVIII in functional assays. Strong inhibition of the FVIII activity in the TG test is consistent with the expected physiological relevance of this “global hemostasis” assay. In turn, our data demonstrate limitations of the classical APTT-based Bethesda assay typically used to analyze FVIII inhibitors in comparison with the CS and TG methods. The discrepant inhibitory effect on the FVIII activity between the assays may indicate a need for optimization or even revising the current methodology of analysis of FVIII inhibitors in Hemophilia A patients. Future studies are needed to evaluate relative clinical relevance of in vitro tests that quantitate activity of the FVIII inhibitory antibodies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.