Abstract
Abstract 4367
Obesity has increased exponentially in recent years and currently a substantial number of children and adolescents are morbidly obese. This development has resulted in the need for bariatric surgery in adolescents. Therefore, a bariatric surgery program has been initiated at Children's National Medical Center (CNMC) and enoxaparin has been used as prophylaxis for venous thromboembolism (VTE). Enoxaparin is the drug of choice for prevention and treatment of VTE in children and adults. The standard of care at CNMC is to dose enoxaparin 40 mg SC q 12 hrs in patients with a BMI < 50 and 60 mg SC q 12 hrs for patients with a BMI > 50 with no routine use of anti-FXa monitoring. Nationally and internationally there are no well defined guidelines for prophylactic doses of enoxaparin and the approach varies among different institutions. This study was therefore designed and conducted to investigate if the current dosing regimens are indeed appropriate.
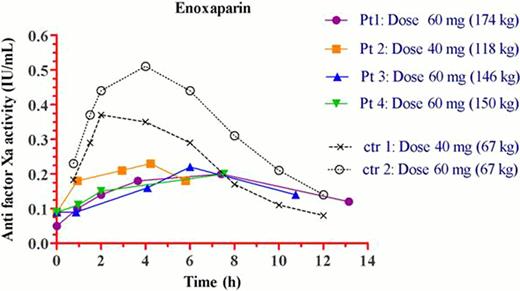
Four morbidly obese adolescents aged 16 to 18 years and with a body weight ranging between 118 and 174 kg were studied. Anti-FXa assay concentrations were collected before (predose) and at 1, 2, 4, 6, and 12 hrs after the administration of the first dose of enoxaparin. In addition, after an extensive literature search, two pharmacokinetic (PK) studies were found that investigated comparable doses of enoxaparin in adult lean healthy volunteers with a mean age of 24 years.
The results are shown in Figure 1 and Table 1. The peak anti-FXa level (Cmax) ranged from 0.2–0.23 IU/ml in the 4 studied adolescents and these values are significantly lower as compared to the range of concentrations (0.38–0.5 IU/ml) detected in the two control groups from the literature. The time to reach Cmax (T max) was delayed, along with a lower area under the time vs concentration curve and the mean residence time.
We conclude, based on our results, that the use of 40–60 mg enoxaparin as the current standard of care for VTE prophylaxis in morbidly obese adolescents results in different and probably insufficient anti-FXa levels as compared to those found in lean patients of comparable age. The clinical significance of this finding still needs to be determined.
| . | Participant-1 (60 mg) . | Participant-2 (40 mg) . | Participant-3 (60 mg . | Participant-4 (60 mg) . | Control group-1* (Mean, SD) . | Control group-2** (Mean SD) . | Control group-2*** (Mean SD) . |
|---|---|---|---|---|---|---|---|
| Tmax (hr) | 7.40 | 4.21 | 6.00 | 7.51 | 3.1 ± 0.4 | 3.0 (median range: 2–5) | 3.5 (median range: 3–6) |
| Cmax (IU/ml) | 0.20 | 0.23 | 0.22 | 0.20 | 0.45 ± 0.05 | 0.38 ± 0.09 | 0.51 |
| AUC (IU/mL) | 2.07 | 1.082 | 1.54 | 1.15 | 3 ± 0.68 | – | – |
| MRT (hr) | 6.66 | 3.20 | 6.42 | 4.54 | 6.7 ± 0.8) | 5.83 ± 0.86 | 6.19 ± 0.74 |
| . | Participant-1 (60 mg) . | Participant-2 (40 mg) . | Participant-3 (60 mg . | Participant-4 (60 mg) . | Control group-1* (Mean, SD) . | Control group-2** (Mean SD) . | Control group-2*** (Mean SD) . |
|---|---|---|---|---|---|---|---|
| Tmax (hr) | 7.40 | 4.21 | 6.00 | 7.51 | 3.1 ± 0.4 | 3.0 (median range: 2–5) | 3.5 (median range: 3–6) |
| Cmax (IU/ml) | 0.20 | 0.23 | 0.22 | 0.20 | 0.45 ± 0.05 | 0.38 ± 0.09 | 0.51 |
| AUC (IU/mL) | 2.07 | 1.082 | 1.54 | 1.15 | 3 ± 0.68 | – | – |
| MRT (hr) | 6.66 | 3.20 | 6.42 | 4.54 | 6.7 ± 0.8) | 5.83 ± 0.86 | 6.19 ± 0.74 |
Azizi et al 40 mg,
Frydman et al 40 mg,
Frydman et al 60 mg.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.