Abstract
Abstract 4340
Myelodysplastic syndrome (MDS) is a malignant hematological disease that comprises a heterogeneous group of clonal hematopoietic stem and progenitor cell disorders, with peripheral cytopenias, bone marrow hypercellularity, high-risk of evolving into acute myeloid leukemia (AML). MDS/AML is a special refractory and palindromic AML characterized by poor therapeutic effects and low complete response rate, as well as high treatment-related complications and mortality. Patients with MDS/AML are often elders and represent more intolerance to routine or intensive chemotherapies. Homoharringtonine, an alkaloid found as the major active component in Chinese plants cephatotaxus fortuneif., has been widely used in AML since the 1970s in China. Decitabine, a hypomethylating agent, is active and has been approved for the treatment of myelodysplastic syndrome (MDS) in recent years.
In order to compare the efficacy, toxicity and long-term prognosis of two chemotherapies HA (Homoharringtonine and cytarabine) and Decitabine regiment in MDS/AML.
A total of 26 MDS/AML patients consisting of 14 males and 12 females were included in this study. They were randomly assigned to receive either HA (H 4mg.d−1,d1–3; A 100mg.m−2d−1, d1–7) or decitabine£.. 20mg.m−2d−1, d1–5£© The effect measures used were hazard ratios (HR) for overall survival (OS), progression-free survival (PFS) and freedom from first progression. Relative risks were used to analyse complete response rate, total response rate, treatment-related mortality and adverse events. A Log-rank test was used in survival analysis, and a Chi-square test was performed for other outcomes.
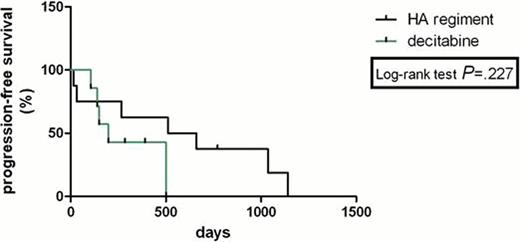
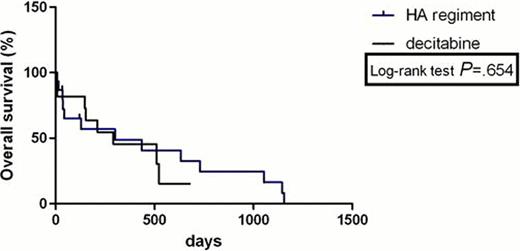
The complete remission (CR) rate with HA regimen according to MDS/AML criteria was 33% and 36% with decitabine (P>0.05). HA group had no lower total response rate than Decitabine group (53% versus 64%, P>0.05). The freedom from first progression in chemotherapy with HA regiment and decitabine was 20% and 18% (P>0.05), respectively. PFS was not statistically significantly longer for two comparators with HR was 0.41(95% confidence interval (CI) 0.09722 to 1.740). There was no statistically significant difference in OS between the HA group and decitabine group with HR was 0.799 (95% CI 0.2992 to 2.133); median survival: 300 days vs 291 days (P>0.05,95% confidence interval (CI) 0.6165 to 1.445). The treatment-related mortality was 13% with HA regimen versus 18% with decitabine at 3 weeks (P>0.05) and 40% with HA regiment versus 18% with decitabine at 3 months (P>0.05). The haematological toxicities and liver function lesion WHO grade III or IV were not significantly higher in the HA group than that in the decitabine group (P>0.05). The total secondary infection rates in all sections of chemotherapies were 58% and 19% (P=0.005) in the two groups, respectively. Secondary infection rate was significantly lower in the decitabine group than that in the HA group.
This analysis showed that Homoharringtonine and cytarabine regiment in treating MDS/AML has a similar therapeutic effect and long-term benefit with decitabine, both regiments were associated with relatively safe and effective outcomes in patients with MDS/AML. However, HA regiment shows a higher risk of secondary infection than decitabine. Longer follow-up and further studies will evaluate prospectively the results of HA regiment versus decitabine in this setting.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.