Abstract
Abstract  4204
4204
Myeloablative allografting induces high rates of persistent molecular remissions (MR) in multiple myeloma (MM) [Corradini et al, J Clin Oncol 1999] and greatly reduces the risk of relapse. Similar results have also been reported after reduced-intensity conditioning [Kröger et al, Blood abstr.2011]. Long term data on minimal residual disease (MRD) kinetics after tandem autologous-nonmyeloablative allografting (auto-allo) are lacking. We here present the results of MRD analyses by nested qualitative PCR and real time quantitative (RQ) PCR on a series of 26 newly diagnosed MM patients treated with auto-allo on a prospective clinical trial [ClinicalTrial.gov, NCT-00702247, Bruno et al, Blood 2009].
Between 1999 and 2009, 19/26 (73%) stage II-III MM patients, median age 52 years (range 42–65), who had a diagnostic bone marrow (BM) specimen suitable for immunoglobulin heavy-chain gene rearrangement (IGH) sequencing were evaluated for MRD by PCR-based methods. The tandem approach consisted of an autograft with melphalan, 200 mg/m2, followed, upon recovery, by non-myeloablative 200 cGy total body irradiation and allogeneic peripheral stem cell infusion. Post-grafting immunosuppression consisted of mycophenolate mofetil and cyclosporine. BM samples were collected at diagnosis, after autograft, at month 1, 3, 6 after the allograft and then every 6 months. Nested-PCR and RQ-PCR analyses were carried out using IGH patient-specific primers, as previously described [Voena et al, Leukemia 1997; Ladetto et al, Biol Bone Marrow Transpl 2000]. For outcome analysis patients were grouped according to reported criteria [Ladetto et al, Blood abstr.2011]. Briefly, FullMR and StandardMR indicated MRD negativity on two consecutive samples by respectively nested-PCR or RQ-PCR (less sensitive but better standardized, according to European Study Group on MRD detection guidelines [van der Vendel et al, Leukemia 2007]). Clinical complete remission (CCR) required absence of serum and urine M-component by electrophoresis and immunofixation and less than 5% plasma cell infiltration in BM aspirates by flow-cytometry.
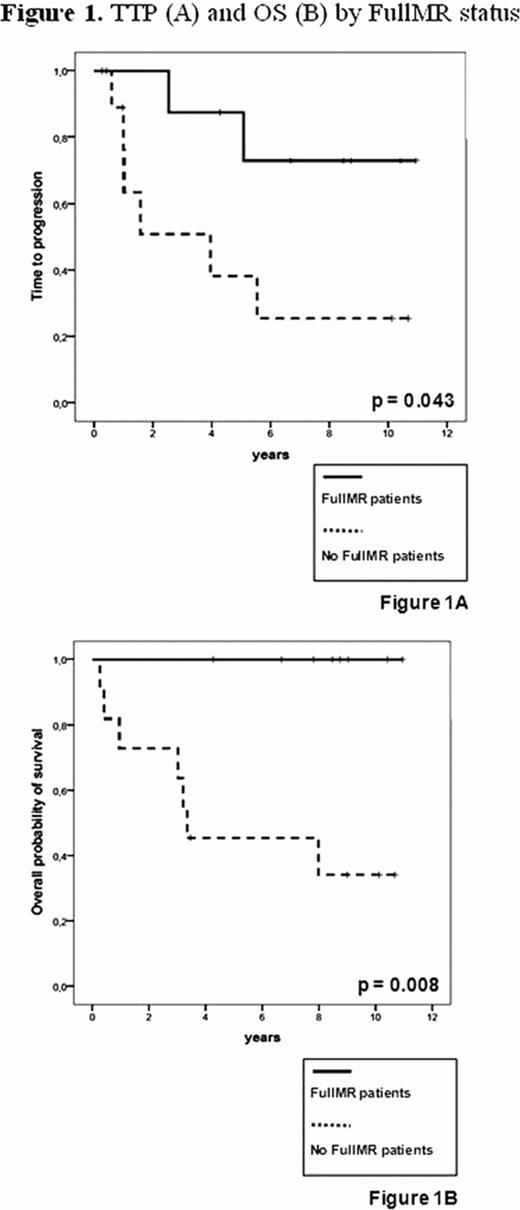
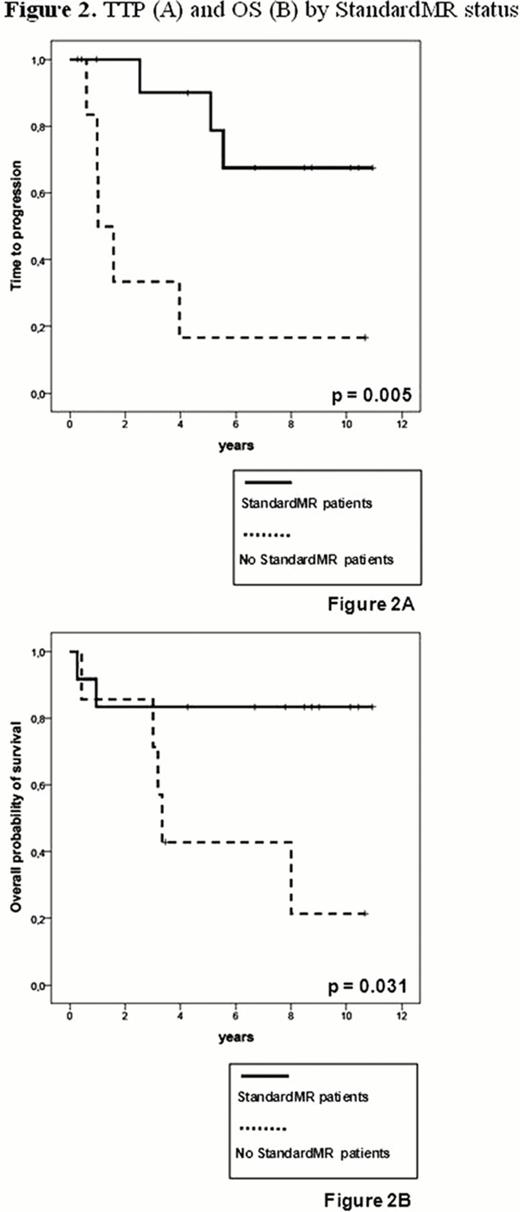
In 19 of 26 patients (73%) a molecular marker was found. In these cases at a median follow-up of 10 years (4.4–12) from diagnosis and 8.9 years (3.5–11) from the allograft, overall survival (OS) was 61% and median time-to-progression (TTP) 5.6 years. Overall, transplant-related mortality occurred in 3/19 patients (16%), while four out of 19 patients (21%) died of disease progression. MRD studies carried out on a total of 148 BM samples showed that after the autograft 3/19 patients (16%) were negative by nested-PCR. After the allograft the rates of PCR-negativity gradually increased up to 4/18 (22%) at 1 and 3 months, 7/17 (41%) at 6 months and 8/15 (53%) at 1 year post-transplant. Overall, 8 patients achieved FullMR at a median time from allograft of 6 months (1–12) and for a median duration of 33 months (6–102). Overall, 8 relapses were observed, 6 in the 11 patients who never achieved FullMR and 2 in patients who reached FullMR: of these one showed a molecular relapse with persistent CCR after sustained FullMR of 3 years and the other relapsed 6 months after last PCR-negative sample. Patients in FullMR had a significantly better median TTP (not reached vs 1.6 years, p=0.043) and OS (not reached vs 3.3 years, p=0.008) than patients who did not achieve FullMR (Fig. 1). StandardMR occurred in 12/19 patients (63%) during the first 24 months post-transplant, at a median time of 2 months (1–18) and for a median duration of 27 months (3–102). Patients in StandardMR showed a significantly better median TTP (not reached vs 1 year, p=0.005) and OS (not reached vs 3.3 years, p=0.031) as compared to patients who not achieved StandardMR (Fig. 2). All patients in either FullMR or StandardMR were also in CCR. There was no significant correlation between occurrence of chronic graft-vs-host disease and MR, suggesting specific graft-vs-myeloma effects.
Auto-allo approach in newly diagnosed MM induces high rates of prolonged FullMR and StandardMR (up to 50%), similar to those reported after myeloablative allografting. MR significantly associated with better TTP and OS and increased during the first year post-allografting, clearly documenting the existence of an effective and persistent graft-vs-myeloma effect, potentially curative in a subset of patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract