Abstract
Abstract  3580
3580
Bortezomib is a 26S proteasome inhibitor that is effective for the treatment of multiple myeloma and indolent lymphomas in adults. Bortezomib at a dose of 1.3 mg/m2 is well tolerated in pediatric patients as a single agent and in combination with cytotoxic chemotherapy. In vitro studies indicate that the proteasome inhibitor bortezomib sensitizes bulk myeloid leukemia and leukemia initiating cells (LIC) to chemotherapy. This Phase 2 study examined the tolerability and efficacy of bortezomib combined with reinduction chemotherapy for relapsed pediatric acute myeloid leukemia (AML).
This study was a two-arm, non-randomized, open label clinical trial in which patients received bortezomib twice weekly (1.3 mg/m2 on days 1, 4, and 8) in a 28-day cycle. Arm A (for patients with prior anthracycline exposure <400mg/m2) combined bortezomib with idarubicin (12 mg/m2 on days 1–3) and cytarabine (100mg/m2 on days 1–7). Arm B (for patients with prior anthracycline exposure >400 mg/m2) combined bortezomib with cytarabine (1000mg/m2 q12h on days 1–5) and etoposide (150 mg/m2on days 1–5). Arm B included an initial dose finding phase (n=12) which determined that bortezomib at 1.3 mg/m2/dose was safe to combine with cytarabine/etoposide. Bortezomib was then tested in a 2-stage efficacy phase for both arms. A second cycle of protocol therapy was allowed for patients with a response of at least stable disease.
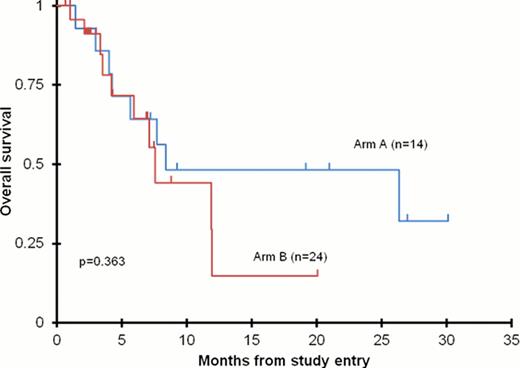
The clinical trial enrolled a total of 52 patients for a total number of 61 cycles of therapy. Arm A enrolled 18 patients (16 eligible, 14 evaluable) and Arm B enrolled 34 patients (n=12 dose finding phase, n=22 efficacy phase (21 evaluable)) for a total of 24 patients evaluable for efficacy in Arm B. The addition of bortezomib to chemotherapy was tolerable in both arms. Severe toxicities included febrile neutropenia (29%) and transient hypokalemia (29%). Although tolerable, both Arm A and Arm B were closed after stage 1 of a two stage study design for failure to meet the efficacy threshold. Arm A response rate was 28% (3 CR and 1 CRp) and Arm B response rate was 42% (8 CR and 2 CRp). Although bortezomib was tolerated in both arms, response rates were adversely affected by a delay in neutrophil count recovery (ANC). Four patients in Arm A and 1 patient in Arm B had a complete response with delayed neutrophil recovery (CRi) which was considered a treatment failure in the study design. If a response of CRi had been included as a treatment success, both Arms A and B would have continued into the second stage of efficacy assessment. The 2-year overall survival (OS) for all evaluable patients was 35 ± 21%, and there was no difference in OS between the two treatment arms (Figure 1).
Overall survival in the 38 evaluable patients enrolled on AAML07P1 combining bortezomib with either idarubicin/low-dose cytarabine (Arm A) or bortezomib with high-dose cyatarabine (1g/m2/dose) and etoposide.
Overall survival in the 38 evaluable patients enrolled on AAML07P1 combining bortezomib with either idarubicin/low-dose cytarabine (Arm A) or bortezomib with high-dose cyatarabine (1g/m2/dose) and etoposide.
Bortezomib is tolerable when added to chemotherapy regimens for children with relapsed/refractory or treatment-related AML. The overall response to the bortezomib containing chemotherapy regimens in the patients enrolled on the efficacy phase (CR + CRp + CRi) (50%) was similar to other salvage regimens for childhood acute myeloid leukemia. Bortezomib, however, did not improve either the CR rate or the OS of this patient cohort.
Off Label Use: Testing of bortezomib for pediatric AML in the context of a phase 2 clinical trial.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract