Abstract
Abstract 3131
Patients with relapsed or refractory Hodgkin's Lymphoma (HL) who fail autologous transplantation seldom survive long term and most have exhausted effective therapeutic options. Preliminary data however suggests a role for allogeneic transplantation (allo-SCT) using reduced intensity conditioning (RIC), with 2–5 year progression free survival (PFS) and overall survival (OS) of 20–36% and 28–64%, respectively. Allo-SCT for HL is still uncommon however with CIBMTR data indicating a median of only 2 allografts/center/year (range: 1–13) at US centers.
We report 7 year outcome on 31 consecutive patients with HL who failed an autograft who were prospectively enrolled in a single institution trial and treated with BEAM RIC regimen (BCNU 300mg/m2 day -6, Etoposide 100mg/m2 daily and Cytarabine 100mg/m2 BID days -5 to -2, and Melphalan 140mg/m2 day -1). Graft vs host disease (GVHD) prophylaxis was with tacrolimus (0.03mg/kg/day starting day -2) and methotrexate (5mg/m2 days +1,+3,+6). Antithymocyte globulin (ATG 1.5mg/kg/day on days -4, -3) was used in 17 patients who underwent matched unrelated donor (MUD) transplantation and had no cytotoxic chemotherapy in the preceding three months.
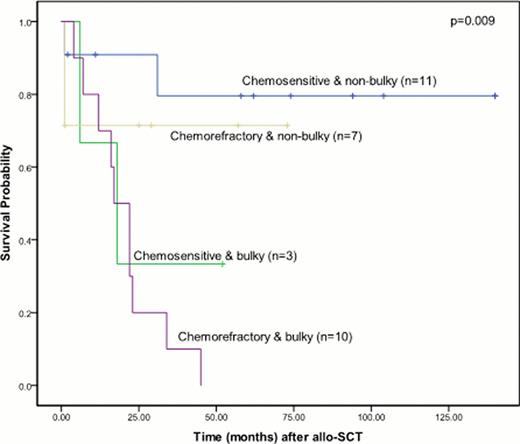
Median time from diagnosis to allo-SCT was 51 months (range: 17–172) and median age was 36 (range: 21–55 years). Patients were heavily pretreated with a median number of 5 prior regimens (including autograft; range: 4–9). Thirteen patients received stem cells from an HLA-matched sibling, 12 MUDs, 5 mismatched unrelated and 1 had mismatched related stem cells. All patients engrafted promptly at a median of 15 days (range: 12–26) defined as an absolute neutrophil count of >0.5 × 109/L and a median of 26 days (range: 10–76) for unsupported platelets >20 × 109/L. 100-day chimerism results were available for 26 patients: 23 (88%) were full donor chimeras (>98% donor DNA), 1 was a mixed chimera at 96% donor DNA, and one patient had graft rejection. The incidence of grade 1–2 acute GVHD was 29% and grade 3–4 was 7%. Incidence of limited stage chronic GVHD (cGVHD) was 56% and extensive stage was 9%. At a median follow up of 7 years the PFS is 36% (95% CI 19–54%) and OS is 42% (95% CI 23–59%), with no relapses seen after 36 months. Non-relapse mortality at one year was 13% (95% CI 5–32%). Significant predictors of improved survival in univariate analysis included chemosensitivity, bulk of disease <2cm, and performance status of 0–1. In multivariate analysis only bulk of disease predicted outcome. Patients transplanted with minimal bulk (<2cm; n=18) had a 4 year PFS and OS of 62% and 75% vs 8% and 8% for patients with bulky disease (p=0.005 for PFS, p=0.001 for OS). To underscore importance of bulk of disease, patients with chemorefractory but non-bulky disease had a favorable 4 year OS of 71% while patients with chemosensitive but bulky disease had a 4 year OS of 33%. Patients with both chemorefractory and bulky disease had the worst outcome with a 100% relapse rate and no survivors after 4 years (Fig 1). Patients with cGVHD had a trend towards lower relapse rates, 43% vs 70% at 4 years (p=0.125). Five patients were treated with donor lymphocyte infusions for relapsed disease after allo-SCT, only 3 achieved a transient PR. Brentuximab vedotin was used in five patients to facilitate allo-SCT, 4 responded (3 PR and 1 CR). Two additional patients who relapsed after allo-SCT received brentuximab vedotin; they are the only survivors with relapsed disease at 29+ and 94+ months, respectively.
RIC BEAM allogeneic transplantation is safe and effective in producing long term remissions in patients with advanced HL who fail autografts. Bulk of disease is the only significant prognostic factor for a successful outcome regardless of chemosensitivity, thus patients with chemorefractory disease should be offered an allo-SCT after additional therapy has been given to achieve a minimal residual disease state, e.g with brentuximab vedotin and/or involved field radiation. While very preliminary, brentuximab vedotin also appears promising in the treatment of relapsed HL after allograft failure and thus should be further explored as maintenance therapy after engraftment to reduce early relapses while awaiting a potential graft vs Hodgkin's effect.
Rodriguez:Seattle Genetics, Inc: Research Funding. Smith:Seattle Genetics, Inc: Consultancy, Research Funding. Stiff:Seattle Genetics, Inc: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.