Abstract
Abstract 2618
AML is a malignant proliferative disorder in which blast stage cells fail to terminally differentiate and accumulate in the blood and bone marrow. AML represents a significant health risk in the general population with rising incidence in aging adults. Standard AML therapy requires intensive combination chemotherapy with a low rate of durable remission, often requires stem cell transplantation, and is associated with significant treatment-related toxicity, especially in elderly patients. Thus, many elderly patients with AML do not receive therapy with curative intent. New therapeutic options for the treatment of AML are urgently needed. In this study, we present evidence that the human protein angiocidin may have the potential for development as a novel, non-toxic therapy for AML. Angiocidin was discovered, cloned and expressed in E. coli in our laboratory (GT) and previously studied for its anti-angiogenic and solid tumor suppressive activity.
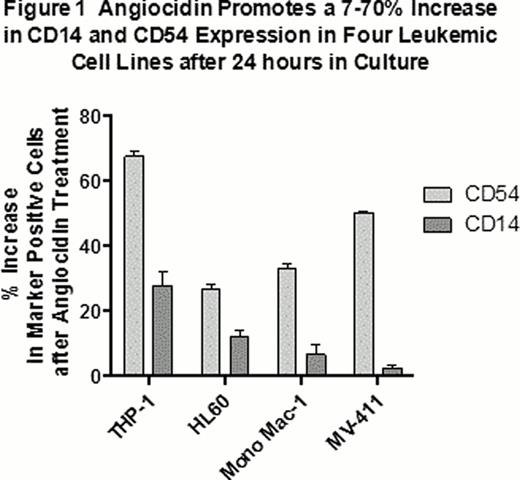
Four experimental AML cell lines (THP-1, Mono-mac-1, HL-60 and MV-411; Figure 1) and 5 of 10 primary human AML samples (Table 1) showed evidence of differentiation when cultured in vitro for 24 hours with 10 μg/mL angiocidin. Differentiation was assessed by flow cytometry measuring the increase in expression of cell surface markers characteristic of normal macrophages. Three macrophage markers were evaluated: CD14, a major receptor for the innate immune system, CD15, a neutrophil phagocytosis receptor, and CD54 or ICAM-1, the major adhesion receptor used for endothelial transmigration. We found that angiocidin up-regulated these macrophage receptors by more than 10-fold in 7–70% of the respective AML cell populations, attesting to its capacity to differentiate AML cell lines and primary human AML samples into macrophage-like phenotypes.
We next evaluated the effect of angiocidin on a xenotransplanted primary human AML sample engrafted in NSG mice. In this model, 5 million patient 983 (see Table 1) mononuclear cells (>80% blasts) were injected iv into previously irradiated NOD/LtSz-scid IL2Rgc null (NSG) mice and allowed to engraft. After 14 weeks, engraftment was measured by flow cytometry of bone marrow aspirates from each animal and found to range between 5–100%. Four treatment groups of 6 animals per group were established with similar distributions of engraftment. Animals were treated over a 4-week period following the 14-week engraftment period: the control group received no treatment; the Ara-C monotherapy group received 40 mg/kg ip qd × 5 at the start of the treatment period; the angiocidin monotherapy group received 3 mg/kg iv biw (M/Th) × 4; the Ara-C + angiocidin combination group was treated with both agents at the same dose levels/routes/schedules as for the respective monotherapy groups. At the end of the 4-week treatment period, bone marrow was harvested from the femurs and tibias of animals (4 bones/animal). Human leukemic cells were identified as those positive for CD45 and CD33 human cell surface markers and were counted by flow cytometry. Figure 2 summarizes the average CD45+/CD33+ cell count from the four treatment groups at the end of the treatment period. Angiocidin monotherapy reduced the human AML burden in bone marrow by 63% relative to control. Angiocidin + Ara-C combination therapy reduced human AML in bone marrow by 79%. That angiocidin's activity is at least additive to that of Ara-C is noteworthy. Consistent with previous observations, angiocidin had no adverse effect on weight or clinical observations in non-engrafted NSG mice at doses up to 3 mg/kg iv tiw × 1.
We believe the combination of in vitro data supporting the capacity of angiocidin to drive differentiation in multiple AML cell lines and primary human AML samples and its activity in a xenotransplantation model that reproduces the genetic diversity of human disease is significant. These observations support the continued evaluation and development of angiocidin as a potential novel, non-toxic therapy for AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.