Abstract
Abstract 238
Tumor lysis syndrome (TLS) is a potentially life-threatening metabolic disorder that most often develops after cytotoxic therapy for hematologic malignancies and rapidly proliferating or large tumors. Rapid lysis of tumor cells may result in hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia, leading to renal dysfunction, cardiac arrhythmia, seizures, and potentially death (Cairo et al Br J Haematol 2004). Acute TLS requires rapid and aggressive intervention focused on hydration and reducing uric acid levels. Despite optimal care, severe acute kidney injury may occur, requiring renal supportive therapy and delay of subsequent chemotherapy cycles (Cairo et al Br J Haematol 2011, Cairo et al Br J Haematol 2012). Prevention of TLS may be more cost-effective than treatment if accurate prediction of patients at risk is possible.
In an effort to assess the risk of treatment-related TLS in large metropolitan hospitals, incidence data from the Henry Ford Health System, obtained between October 31, 2000 and June 30, 2011, were analyzed retrospectively.
Patients included in the analysis were those newly diagnosed with cancer, were chemotherapy naïve at baseline, had their initial chemotherapy administration within 7 days of their cancer diagnosis, and had no prior history of TLS (N=951; Figure 1). The majority of patients were Caucasian (60%); slightly more than half were female (52%), and the median age at cancer diagnosis was 67 years.
Laboratory TLS (LTLS) and clinical TLS (CTLS) were defined by using a modified Cairo-Bishop classification (Cairo et al Br J Haematol 2004). Patients who had 2 or more abnormal laboratory values (serum uric acid, potassium, phosphorous, or calcium) reported on the same day within 3 days before to 7 days after anticancer therapy were identified as having LTLS. Patients with LTLS who had ≥1 clinical complication (renal insufficiency, cardiac arrhythmia, seizure, or sudden death) within 5 days of an abnormal laboratory value were identified as having CTLS.
Preliminary descriptive results indicated that the overall incidence was 9.3% for LTLS and 6.7% for CTLS (Fig 2). TLS rates were highest for hematologic cancers (21.1% for LTLS, 15.1% for CTLS) followed by gastrointestinal (8.4% for LTLS, 5.0% for CTLS), epithelial (4.9% for LTLS, 4.0% for CTLS), and genitourinary cancers (3.8% for LTLS, 3.3% for CTLS). Among hematologic cancers, rates were highest for acute leukemia (26.0% for LTLS, 16.4% for CTLS) and multiple myeloma (21.7% for LTLS, 20.3% for CTLS). Among solid tumors, rates of TLS were highest for esophageal (23.5% for LTLS, 5.9% for CTLS), liver (14.3% for LTLS and CTLS), and endocrine tumors (11.8% for LTLS and CTLS).
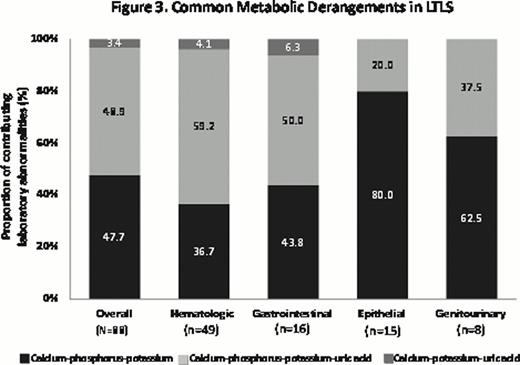
Across hematologic cancer types, approximately two-thirds of patients with LTLS developed CTLS. The rate of LTLS was highest in acute leukemia patients (26%) followed by multiple myeloma (21.7%), whereas multiple myeloma patients had the highest proportion of CTLS (20.3% for multiple myeloma vs 16.4% for leukemia). In examining the abnormal laboratory values used to determine LTLS, 52% of patients had hyperuricemia (Fig 3). Rates of hyperuricemia by cancer type were approximately 63% for hematologic, 56% for epithelial, 38% for genitourinary, and 20% for gastrointestinal cancers. For CTLS, the majority of patients had renal insufficiency or creatinine elevations (62.5%), followed by arrhythmia (35.9%), and seizure (1.6%).
Findings suggest that hyperuricemia is not the sole contributor to treatment-related LTLS—hyperkalemia, hyperphosphatemia, and hypocalcemia were also evident in a substantial proportion of patients with LTLS. Limitations of the study include a restrictive exclusion of patients who do not initiate chemotherapy within 1 week of diagnosis, thereby eliminating non-acute TLS cases and possibly patients at low- to intermediate risk for developing TLS.
This preliminary analysis will be the basis for refining the methodology for examining LTLS and CTLS incidence across a broad array of cancer types in a large population database. Such information may be used by health care providers to more accurately predict the risk of patients developing TLS.
Thompson:Sanofi-Aventis: Employment. Stern:Sanofi: Consultancy. Sherman:Sanofi: Consultancy.
Funding for this study and publication were provided by Sanofi.
Author notes
Asterisk with author names denotes non-ASH members.