Abstract
DEP-1/CD148 is a receptor-like protein tyrosine phosphatase with antiproliferative and tumor-suppressive functions. Interestingly, it also positively regulates Src family kinases in hematopoietic and endothelial cells, where we showed it promotes VE-cadherin–associated Src activation and endothelial cell survival upon VEGF stimulation. However, the molecular mechanism involved and its biologic functions in endothelial cells remain ill-defined. We demonstrate here that DEP-1 is phosphorylated in a Src- and Fyn-dependent manner on Y1311 and Y1320, which bind the Src SH2 domain. This allows DEP-1–catalyzed dephosphorylation of Src inhibitory Y529 and favors the VEGF-induced phosphorylation of Src substrates VE-cadherin and Cortactin. Accordingly, RNA interference (RNAi)–mediated knockdown of DEP-1 or expression of DEP-1 Y1311F/Y1320F impairs Src-dependent biologic responses mediated by VEGF including permeability, invasion, and branching capillary formation. In addition, our work further reveals that above a threshold expression level, DEP-1 can also dephosphorylate Src Y418 and attenuate downstream signaling and biologic responses, consistent with the quiescent behavior of confluent endothelial cells that express the highest levels of endogenous DEP-1. Collectively, our findings identify the VEGF-dependent phosphorylation of DEP-1 as a novel mechanism controlling Src activation, and show this is essential for the proper regulation of permeability and the promotion of the angiogenic response.
Introduction
DEP-1/CD148 (also called PTPη or PTPRJ) is a receptor-like protein tyrosine phosphatase (PTP) expressed in several cell types including epithelial, endothelial, and hematopoietic cells.1 It encompasses an extracellular domain containing 8 fibronectin-type III-like motifs, a transmembrane domain, a single intracellular catalytic domain, and a short C-terminal tail.2 Initial studies demonstrated that its expression increases with cell density and suggested a function in cell contact–mediated growth inhibition.2,3 Overexpression of DEP-1 in cancer cells was also reported to inhibit their growth, while thyroid cell transformation was associated with its decreased expression, indicative of a role for DEP-1 as a tumor suppressor.4-8 DEP-1 was further identified as the gene associated with the mouse colon cancer susceptibility locus (Scc1), and was found to be frequently deleted and mutated in human cancers.9 These growth inhibitory functions of DEP-1 are consistent with the nature of some of its reported substrates, which include the PDGF-β, HGF (Met), and VEGF (VEGFR2) receptors as well as Src family kinases (SFKs), ERK1/2, and the p85 subunit of PI3K.10-18 DEP-1 also dephosphorylates proteins from the cell-cell junctional complexes including p120catenin, occludin, and ZO-1, which might impact biologic functions dependent on the loosening/strengthening of intercellular contacts.11,14,19
VEGFR2 is a potent activator of the angiogenic response and is the main mediator of the mitogenic, chemotactic, permeability, and survival effects of VEGF in normal and tumor-associated vessels.20 VE-cadherin adhesion complexes are important sites of VEGF-dependent signaling in confluent cells, as activated VEGFR2 associates to these complexes to mediate Akt activation and cell survival.21 DEP-1 was shown to colocalize to these sites and to attenuate the phosphorylation of VEGFR2, resulting in the impaired activation of ERK1/2 and the contact inhibition of endothelial cell proliferation.13,22 However, DEP-1 was also reported to positively regulate VE-cadherin–associated Src and Akt and to promote VEGF-dependent endothelial cell survival.15 Consistent with these distinct in vitro functions, inactivation of DEP-1 in mice via the swapping of its catalytic domain and C-terminal tail with GFP revealed both positive and negative regulatory effects in vascular development, and resulted in defective vessel remodeling and branching in addition to increased endothelial cell proliferation.23
SFKs promote the remodeling of cell-cell junctions, cell invasion, and permeability,24-26 which are essential cellular functions that contribute to vessel sprouting and neovascularization.27 In their inactive state, SFKs adopt a closed conformation where the SH2 domain is linked to the phosphorylated C-terminal tyrosine residue (Y529 in human Src). To become active, the SH2 domain must engage with higher affinity binding sites on other molecules to release the Src C-terminal tyrosine residue and allow its dephosphorylation by PTPs (phospho-displacement mechanism). This then induces conformational changes that result in transphosphorylation of the catalytic tyrosine residue (Y418 in human Src) and SFK activation.28 DEP-1 has been shown to dephosphorylate Src Y529 and Y418 in vitro, but to preferentially target Y529 and activate Src when overexpressed in thyroid tumor cells.12 Conversely, the silencing of DEP-1 in endothelial cells results in the increased phosphorylation of VE-cadherin–associated Src on Y529, consistent with the observed inhibition of its activity and decreased Y418 phosphorylation in response to VEGF.15 Recent genetic studies demonstrated that DEP-1/CD148 is also an important activator of SFKs in hematopoietic cells, and promotes immunoreceptor signaling, B-cell and myeloid lineage development, phagocytosis, as well as platelet activation and thrombosis.1,18,29
Despite their broad implication in the activation of SFKs, the molecular mechanism underlying the ability of most PTPs to activate Src remains incompletely understood. The phosphorylation of receptor-type PTPϵ and PTPα has been suggested to regulate their ability to activate Src.30-32 We show here that VEGF induces the tyrosine phosphorylation of DEP-1 on Y1311 and Y1320 in endothelial cells. This is essential for Src Y529 dephosphorylation and the promotion of Src-dependent angiogenic responses.24,25,33 However, we also found that when expressed above a threshold level, such as that observed in confluent and quiescent cells, DEP-1 rather inhibits Src and downstream responses. This study therefore identifies for the first time regulatory events allowing the control of DEP-1–mediated Src activation and further defines the molecular mechanisms underlying the promotion of VEGF-dependent permeability and angiogenesis.
Methods
Antibodies, reagents, DNA constructs, and cell-culture conditions
Details are available in supplemental Methods and Figures (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
DNA transfection, cell stimulation, and lysis
HEK 293 and HEK 293T cells were seeded at 0.8 and 1.2 × 106 cells/10-cm dish, respectively, and transfected using the standard calcium phosphate method. Cells were lysed 48 hours after transfection in a 50mM HEPES (pH 7.5) buffer,15 as for all other experiments described below. Mouse embryonic fibroblasts (MEFs; 1.7 × 106 cells/10-cm dish) were transfected in OptiMEM (Invitrogen) using Lipofectamine 2000. Bovine aortic endothelial cells (BAECs; 3.3 × 104 cells/cm2) and human umbilical vein endothelial cells (HUVECs; 2.5-3.0 × 104 cells/ cm2) were transfected in OptiMEM with Lipofectamine 2000 and Lipofectin, respectively (see supplemental Methods and Figures for amounts of DNA used). Medium was replaced 16 hours later with supplemented DMEM (BAECs) or M200 (HUVECs). Cells were serum-starved the next day for 16 hours (BAECs), or 42 hours later for 6 hours (HUVECs), and then stimulated with VEGF 50 ng/mL for the indicated times before cell lysis. Alternatively, HUVECs were collected for biologic assays.
siRNA transfection
HUVECs (3 × 104 cells/cm2 for cell-signaling experiments and 2.2-2.5 × 104 cells/cm2 for biologic assays) were transfected with DEP-1 (Hs_PTPRJ_3_HP) and AllStars control siRNAs (QIAGEN) at a final concentration of 75nM in M200 medium using Dharmafect reagent 4 (Dharmacon). Medium was replaced 16 hours after transfection with supplemented M200. At 42 hours after transfection, cells were serum-starved for 6 hours in M200 and stimulated with VEGF as described in the previous paragraph. Alternatively, cells were submitted to biologic assays.
Immunoprecipitation, Western blotting, GST pull-down, in vitro association assay, and immunofluorescence
Details to these procedures are available in supplemental Methods and Figures.
Capillary-like formation on Matrigel
Ice-cold Matrigel (BD Biosciences; 50 μL/well) was added to flat-bottom 96-well plates and allowed to solidify 1 hour at 37°C. HUVECs (2 × 104 cells/well) were seeded in duplicates on Matrigel and incubated 5-6 hours at 37°C in supplemented M200. Capillaries were fixed in phosphate-buffered formalin.
Matrigel invasion
Transwell filters (polycarbonate membrane, 8-μm pore size; Corning Brand, Fisher) were coated with 50 μL of Matrigel (2 mg/mL). Transfected HUVECs or BAECs (5 × 105 cells) were resuspended in 400 μL of M200 medium or DMEM, respectively, and seeded in duplicate in the top chamber. The lower chamber was filled with medium containing VEGF (50 ng/mL). Cells were incubated for 24 hours and then fixed with phosphate-buffered formalin before staining with crystal violet (0.1% in 20% [v/v] methanol). The Matrigel layer and cells remaining on top of the filter were wiped off. Only cells that went through the filter pores were visualized and counted.
Endothelial permeability assay
Six hours after transfection, HUVECs were seeded on Transwell permeability inserts (6.5-mm diameter, 1.0-μm pore size; BD Falcon, BD Biosciences) precoated with rat tail collagen type I (50 μg/mL; Sigma-Aldrich). After 46 hours, DNA- or siRNA-transfected cells were serum-starved for 1 hour or 2 hours, respectively, in M200 medium and then stimulated with VEGF (50 ng/mL) in the presence of FITC-dextran (40-kDa size, 1 mg/mL; Sigma-Aldrich). Permeability was determined by measuring the fluorescence at 520 nm (498 nm excitation) that was emitted from 50-μL medium aliquots taken from the bottom chambers using a Victor3 V fluorescence reader (PerkinElmer).
In vitro phosphatase assay
Immunoprecipitated DEP-1 was resuspended in 50 μL of a 50mM HEPES (pH 7.4) assay buffer containing BSA (0.1 mg/mL) and DTT (3mM) and transferred into a 96-well plate. Paranitrophenyl phosphate (pNPP; 50 μL of a 50mM stock in water) was added to the wells and incubated 5-15 minutes at room temperature. Absorbance was read at 405 nm with a Victor3 V fluorescence reader (PerkinElmer).
Data analysis
Statistical significance was evaluated with the Mann-Whitney rank sum test using SPSS software. P values of less than .05 were considered to be significant.
Results
DEP-1 is tyrosine-phosphorylated in a Src family kinase-dependent manner
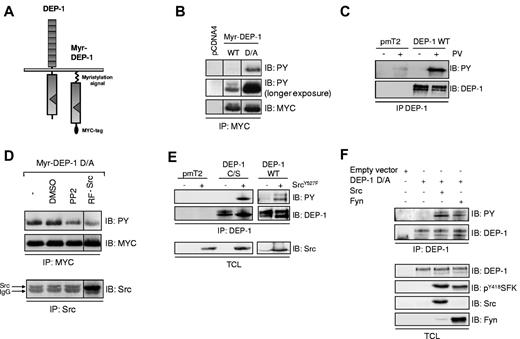
Activation of Src by the protein tyrosine phosphatase PTPϵ has been linked to its tyrosine phosphorylation and its capacity to associate with the kinase.31,34 To determine whether a similar mechanism was involved in the ability of DEP-1 to activate Src, we first confirmed that DEP-1 was tyrosine phosphorylated and identified the kinases potentially involved. We found that WT Myr-DEP-1 and a catalytically inactive and substrate trapping mutant (Myr-DEP-1 D/A), which encompass the intracellular portion of DEP-1 fused to the myristylated sequence of Src, were constitutively phosphorylated when expressed in HEK 293 cells (Figure 1A-B). However, the phosphorylation of WT Myr-DEP-1 was much weaker, indicating that it was subject to autodephosphorylation, similarly to what was observed for PTPϵ.31 Consistent with these observations, the phosphorylation of full-length WT DEP-1 was enhanced when cells were incubated with pervanadate, a general PTP inhibitor (Figure 1C). To determine whether SFKs were implicated in this constitutive phosphorylation event, HEK 293 cells expressing Myr-DEP-1 D/A were treated with a pharmacologic SFK inhibitor (PP2), DMSO as vehicle control, or cotransfected with a dominant-negative mutant form of Src (RF-Src). As shown in Figure 1D, PP2 treatment or expression of RF-Src decreased the tyrosine phosphorylation of DEP-1, while cotransfection of a constitutively active Src mutant (Src Y527F) with either full-length WT DEP-1 or the DEP-1 C/S catalytically inactive mutant increased their phosphorylation (Figure 1E). Note that compared with DEP-1 D/A, DEP-1 C/S has virtually no ability to trap substrates,15 and thus is not similarly protected from dephosphorylation by other endogenous phosphatases.35 Lastly, Figure 1F shows that phosphorylation of full-length DEP-1 D/A is barely detectable in Src/Yes/Fyn-null mouse embryonic fibroblasts (SYF MEFs), but rescued by reexpression of WT Src or Fyn. These results thus demonstrate that DEP-1 can be tyrosine-phosphorylated in a SFK-dependent manner.
DEP-1 is tyrosine phosphorylated in a SFK-dependent manner. (A) Structure of full-length DEP-1 and Myc-tagged Myr-DEP-1. (B) Myr-DEP-1 WT and inactive Myr-DEP-1 D/A mutant were expressed in HEK 293 cells. Tyrosine phosphorylation of immunoprecipitated DEP-1 was analyzed by immunoblotting (IB) with the PY99 phosphotyrosine antibody (PY). The membrane was stripped and reprobed with the Myc antibody to detect equivalent levels of immunoprecipitated Myr-DEP-1. (C) Tyrosine phosphorylation of full-length WT DEP-1 is increased by pervanadate (PV) treatment of HEK 293T cells transfected with empty vector (pmT2) or full-length WT DEP-1. At 48 hours after transfection, cells were incubated or not with PV (100μM) for 20 minutes before cell lysis. DEP-1 was immunoprecipitated (IP) with the clone 143-41 mouse antibody, and its tyrosine phosphorylation determined by immunoblotting with the PY99 antibody. The membrane was stripped and reprobed with the DEP-1 antibody (clone 143-41) to show similar levels of immunoprecipitated DEP-1. (D) Tyrosine phosphorylation of Myr-DEP-1 D/A is impaired by PP2 or by a Src dominant-negative mutant. HEK 293 cells were transfected with Myr-DEP-1 D/A and incubated with either PP2 (5μM; 30 minutes) or vehicle (DMSO), or cotransfected with a Src dominant-negative mutant (RF-Src). Tyrosine phosphorylation of immunoprecipitated DEP-1 was detected as described in panel B. Src-RF expression was determined after immunoprecipitation with the mouse Src antibody (GD11 clone) and immunodetection with the rabbit clone 36D10. (E) Tyrosine phosphorylation of DEP-1 is increased by coexpression of a constitutively active Src mutant. HEK 293T cells were transfected with empty vector (pmT2), WT DEP-1, or the C/S mutant in the presence or not of active Src Y527F. Tyrosine phosphorylation of immunoprecipitated DEP-1 was determined as described in panel C. Immunoblotting of total cell lysates with the Src antibody (clone GD11) reveals equivalent Src Y527F expression levels. (F) WT Src and Fyn promote DEP-1 D/A tyrosine phosphorylation. SYF MEFs were transfected with empty vector (pmT2), the DEP-1 D/A mutant alone, or in combination with either WT Src- or Fyn-encoding vectors. Phosphorylation of immunoprecipitated DEP-1 is revealed as described in panel C. Immunoblotting of total cell lysates with the indicated antibodies demonstrates similar amounts of DEP-1 and SFKs in the various conditions. All results are representative of 3 independent experiments.
DEP-1 is tyrosine phosphorylated in a SFK-dependent manner. (A) Structure of full-length DEP-1 and Myc-tagged Myr-DEP-1. (B) Myr-DEP-1 WT and inactive Myr-DEP-1 D/A mutant were expressed in HEK 293 cells. Tyrosine phosphorylation of immunoprecipitated DEP-1 was analyzed by immunoblotting (IB) with the PY99 phosphotyrosine antibody (PY). The membrane was stripped and reprobed with the Myc antibody to detect equivalent levels of immunoprecipitated Myr-DEP-1. (C) Tyrosine phosphorylation of full-length WT DEP-1 is increased by pervanadate (PV) treatment of HEK 293T cells transfected with empty vector (pmT2) or full-length WT DEP-1. At 48 hours after transfection, cells were incubated or not with PV (100μM) for 20 minutes before cell lysis. DEP-1 was immunoprecipitated (IP) with the clone 143-41 mouse antibody, and its tyrosine phosphorylation determined by immunoblotting with the PY99 antibody. The membrane was stripped and reprobed with the DEP-1 antibody (clone 143-41) to show similar levels of immunoprecipitated DEP-1. (D) Tyrosine phosphorylation of Myr-DEP-1 D/A is impaired by PP2 or by a Src dominant-negative mutant. HEK 293 cells were transfected with Myr-DEP-1 D/A and incubated with either PP2 (5μM; 30 minutes) or vehicle (DMSO), or cotransfected with a Src dominant-negative mutant (RF-Src). Tyrosine phosphorylation of immunoprecipitated DEP-1 was detected as described in panel B. Src-RF expression was determined after immunoprecipitation with the mouse Src antibody (GD11 clone) and immunodetection with the rabbit clone 36D10. (E) Tyrosine phosphorylation of DEP-1 is increased by coexpression of a constitutively active Src mutant. HEK 293T cells were transfected with empty vector (pmT2), WT DEP-1, or the C/S mutant in the presence or not of active Src Y527F. Tyrosine phosphorylation of immunoprecipitated DEP-1 was determined as described in panel C. Immunoblotting of total cell lysates with the Src antibody (clone GD11) reveals equivalent Src Y527F expression levels. (F) WT Src and Fyn promote DEP-1 D/A tyrosine phosphorylation. SYF MEFs were transfected with empty vector (pmT2), the DEP-1 D/A mutant alone, or in combination with either WT Src- or Fyn-encoding vectors. Phosphorylation of immunoprecipitated DEP-1 is revealed as described in panel C. Immunoblotting of total cell lysates with the indicated antibodies demonstrates similar amounts of DEP-1 and SFKs in the various conditions. All results are representative of 3 independent experiments.
DEP-1 C-terminal Y1311 and Y1320 are phosphorylation sites and associate with Src
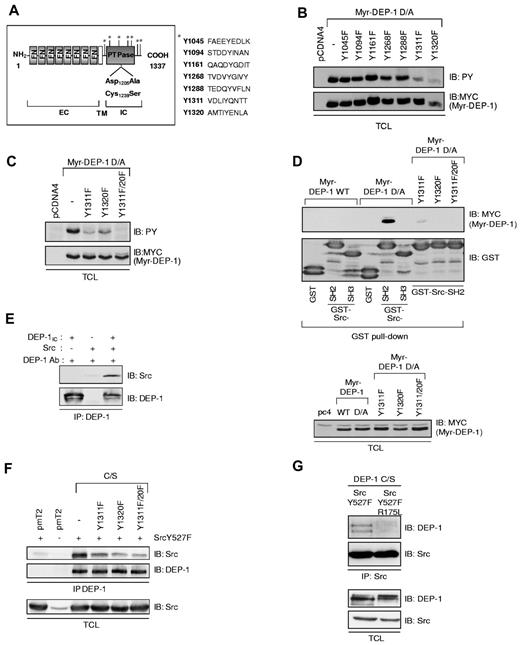
To identify DEP-1 phosphorylated site(s), tyrosine residues corresponding to SFK consensus phosphorylation sites and/or potential SH2-domain binding sites were mutated in Myr-DEP-1 D/A (Figure 2A). Figure 2B reveals that the Myr-DEP-1 D/A Y1311F and Y1320F mutants were the least phosphorylated. Consistently, mutation of both residues led to the complete abrogation of Myr-DEP-1 D/A tyrosine phosphorylation (Figure 2C). These 2 tyrosine residues were the only tyrosine-phosphorylated sites detected by mass spectrometry analysis performed on Myr-DEP-1 D/A (data not shown).
DEP-1 Y1311 and Y1320 are major phosphorylation sites that associate with Src via its SH2 domain. (A) Diagram representing the potential SFK tyrosine phosphorylation sites in the intracellular domain of DEP-1 according to published consensus sequences or Scansite predictions (http://scansite.mit.edu/). FN indicates fibronectin-type III-like repeats; EC, extracellular; TM, transmembrane; and IC, intracellular. (B) HEK 293 cells were transfected with “nonmutated” (−) Myr-DEP-1 D/A or the various Y/F mutants. Their tyrosine phosphorylation was detected by immunoblotting (IB) total cell lysates (TCL) with the PY99 antibody (PY). Expression levels of transfected Myr-DEP-1 were detected with a Myc antibody. (C) HEK 293 cells were transfected with Myr-DEP-1 mutated at both Y1311 and Y1320, which is not detectably tyrosine phosphorylated. (D) DEP-1 associates with the Src SH2 domain via its phosphorylated Y1311 and Y1320 residues. Lysates of HEK 293 cells transfected with the indicated constructs were incubated with GST-Src-SH2, GST-Src-SH3, or with GST alone. Association of DEP-1 with Src domains was detected by immunoblotting with the MYC antibody. The equivalent amount of GST fusion proteins used in the pull-down assay is detected with a GST antibody. (Bottom panel) Lysates of transfected HEK 293 cells were immunoblotted with the MYC antibody to show the similar expression level of DEP-1 constructs. (E) In vitro association of recombinant Src with the purified intracellular domain of DEP-1 (DEP-1IC) previously phosphorylated by Src in vitro. DEP-1IC was immunoprecipitated with DEP-1 goat antibodies and associated Src was immunodetected using the 36D10 clone. (F) The association of DEP-1 C/S and DEP-1 C/S Y/F mutants to Src was investigated in HEK 293T cells cotransfected with constitutively active Src, to induce maximal tyrosine phosphorylation of DEP-1. The coprecipitation of Src with DEP-1 and the equal expression of Src Y527F were detected by immunoblotting with a Src antibody (GD11 clone). (G) Mutation in the Src SH2 domain abrogates its ability to associate with DEP-1. HEK 293T cells were cotransfected with DEP-1 C/S and activated Src Y527F or the Src Y527F/R175L SH2 domain mutant. Src was immunoprecipitated with the mouse GD11 mouse clone, and associated DEP-1 was detected with the goat antibody. Immunoblotting of total cell lysates with the DEP-1 goat antibody or the Src GD11 antibody reveal that equal amounts of Src and DEP-1 constructs were expressed. All results are representative of at least 3 independent experiments.
DEP-1 Y1311 and Y1320 are major phosphorylation sites that associate with Src via its SH2 domain. (A) Diagram representing the potential SFK tyrosine phosphorylation sites in the intracellular domain of DEP-1 according to published consensus sequences or Scansite predictions (http://scansite.mit.edu/). FN indicates fibronectin-type III-like repeats; EC, extracellular; TM, transmembrane; and IC, intracellular. (B) HEK 293 cells were transfected with “nonmutated” (−) Myr-DEP-1 D/A or the various Y/F mutants. Their tyrosine phosphorylation was detected by immunoblotting (IB) total cell lysates (TCL) with the PY99 antibody (PY). Expression levels of transfected Myr-DEP-1 were detected with a Myc antibody. (C) HEK 293 cells were transfected with Myr-DEP-1 mutated at both Y1311 and Y1320, which is not detectably tyrosine phosphorylated. (D) DEP-1 associates with the Src SH2 domain via its phosphorylated Y1311 and Y1320 residues. Lysates of HEK 293 cells transfected with the indicated constructs were incubated with GST-Src-SH2, GST-Src-SH3, or with GST alone. Association of DEP-1 with Src domains was detected by immunoblotting with the MYC antibody. The equivalent amount of GST fusion proteins used in the pull-down assay is detected with a GST antibody. (Bottom panel) Lysates of transfected HEK 293 cells were immunoblotted with the MYC antibody to show the similar expression level of DEP-1 constructs. (E) In vitro association of recombinant Src with the purified intracellular domain of DEP-1 (DEP-1IC) previously phosphorylated by Src in vitro. DEP-1IC was immunoprecipitated with DEP-1 goat antibodies and associated Src was immunodetected using the 36D10 clone. (F) The association of DEP-1 C/S and DEP-1 C/S Y/F mutants to Src was investigated in HEK 293T cells cotransfected with constitutively active Src, to induce maximal tyrosine phosphorylation of DEP-1. The coprecipitation of Src with DEP-1 and the equal expression of Src Y527F were detected by immunoblotting with a Src antibody (GD11 clone). (G) Mutation in the Src SH2 domain abrogates its ability to associate with DEP-1. HEK 293T cells were cotransfected with DEP-1 C/S and activated Src Y527F or the Src Y527F/R175L SH2 domain mutant. Src was immunoprecipitated with the mouse GD11 mouse clone, and associated DEP-1 was detected with the goat antibody. Immunoblotting of total cell lysates with the DEP-1 goat antibody or the Src GD11 antibody reveal that equal amounts of Src and DEP-1 constructs were expressed. All results are representative of at least 3 independent experiments.
To test whether these sites were Src SH2-domain binding sites, we first performed in vitro association experiments and investigated the ability of GST alone, GST-Src-SH2, or GST-Src-SH3 fusion proteins to associate with Myr-DEP-1 WT, Myr-DEP-1 D/A, or the Myr-DEP-1 D/A Y/F mutants from transfected HEK 293 cell lysates. The highly tyrosine-phosphorylated Myr-DEP-1 D/A associated with the GST-Src-SH2 fusion proteins with a strong affinity, while mutation of Y1311 and Y1320 residues abolished this interaction (Figure 2D). We further showed that recombinant Src directly associated with the purified phosphorylated intracellular domain of DEP-1 in vitro (Figure 2E), supporting the conclusion that DEP-1 phosphorylated on Y1311 and Y1320 directly associates with the Src SH2 domain.
To demonstrate that the phosphorylated DEP-1 Y1311 and Y1320 are also implicated in the binding of Src in HEK 293T cells, DEP-1 C/S and the Y/F mutants were coexpressed with constitutively active Src Y527F to enhance the phosphorylation of DEP-1, as previously done (Figure 1E). Figure 2F shows that full-length DEP-1 C/S coimmunoprecipitated with Src. Mutations of Y1311 or Y1320 decreased the association of DEP-1 to Src, with the mutation at Y1320 having a slightly stronger effect. In the converse experiment, DEP-1 C/S failed to associate with a Src SH2 domain mutant (Y527F/R175L), further confirming that DEP-1 associates with Src through its SH2 domain (Figure 2G). Altogether, these results demonstrate that DEP-1 phosphorylated on Y1311 and Y1320 associate to Src via its SH2 domain, and that Y1320 may be a slightly preferred binding site.
Mutation of Y1311 and Y1320 impairs DEP-1–mediated activation of Src in HEK 293T cells, without affecting PTP activity
To determine whether the binding of the Src SH2 domain to phosphorylated DEP-1 Y1311 and Y1320 could be involved in a phospho-displacement mechanism leading to Src activation, we first investigated the ability of WT DEP-1 and the Y/F mutants to activate Src in HEK 293T cells. Figure 3A shows that transfection of increasing amounts of WT DEP-1 led to the progressive dephosphorylation of inhibitory Y529 while increasing the phosphorylation of Y418 and kinase activity. Interestingly, as levels of expression reached a maximum, Y418 phosphorylation and kinase activity also started to decrease, suggesting that DEP-1 can also dephosphorylate Src on Y418 in these conditions, as previously observed in vitro.12 This experiment thus indicated that the level of DEP-1 expression is crucial in determining its substrate specificity and function. We next investigated the consequences of expressing DEP-1 Y/F mutants on Src phosphorylation using optimal transfection conditions resulting in the activation of Src by WT DEP-1, as determined in Figure 3A. Results show that Src Y418 phosphorylation was slightly decreased on expression of the DEP-1 Y1311F mutant compared with cells expressing WT DEP-1, while changes in the phosphorylation status of Src Y529 were barely visible. In contrast, expression of the DEP-1 Y1320F or Y1311F/Y1320F mutants strongly impaired phosphorylation of Y418 and dephosphorylation of Y529, in a manner comparable with that observed after expression of the catalytically inactive DEP-1 C/S mutant (Figure 3B). Using a validated phospho-specific DEP-1 antibody recognizing phosphorylated Y1320 (see supplemental Figure 1), the constitutive phosphorylation of WT DEP-1 and of the Y1311F mutant was detected in HEK 293T cells and correlated with their ability to up-regulate Src Y418 phosphorylation (Figure 3C). The DEP-1 C/S catalytically inactive mutant was also constitutively phosphorylated, demonstrating that tyrosine phosphorylation is not sufficient for Src activation and that the catalytic activity of DEP-1 is also required. These results thus suggest that phosphorylated DEP-1 Y1311 and Y1320 associate with Src, and that this is required for Src activation. However, as phosphorylation of PTPs can modulate their catalytic activity,36 the consequences of mutating Y1311 and Y1320 on the catalytic activity of DEP-1 were also investigated. For this, WT DEP-1 and the various Y/F mutants were cotransfected with activated Src (Y527F) in HEK 293T cells. An in vitro PTP assay using pNPP as a substrate was performed on immunoprecipitated DEP-1. The results reveal that the Y1311F, Y1320F, or double Y1311F/Y1320F mutations did not significantly interfere with the catalytic activity of DEP-1 in these experimental conditions (Figure 3D). To further confirm this result, dephosphorylation of VEGFR2, previously identified as a DEP-1 substrate,13,15 was monitored in VEGF-stimulated HEK 293T cells coexpressing VEGFR2 with either WT DEP-1 or the Y/F mutants in conditions similar to those of Figure 3B. Immunoblotting with a general phosphotyrosine antibody showed that the DEP-1 Y1311F/Y1320F mutant was as efficient as WT DEP-1 at reducing the phosphorylation of VEGFR2, while the C/S mutant had no effect, as expected (Figure 3E). On the basis of these results, we conclude that the phosphorylation of DEP-1 on Y1311 and Y1320 is not involved in the regulation of its catalytic activity. This then strongly suggests that the decreased dephosphorylation/activation of Src in cells expressing the DEP-1 Y1311F/Y1320F mutant is not because of reduced catalytic activity of the mutant but rather to its lost ability to associate with Src.
Mutation of Y1311 and Y1320 impairs DEP-1–mediated activation of Src in HEK 293T cells, without affecting PTP activity. (A) HEK 293T cells were transfected with increasing amounts of WT DEP-1 cDNA vector. The phosphorylation level of Src on Y529 and Y418 was determined by immunoblotting total cell lysates (TCL) with the corresponding phospho-specific antibodies. (Bottom panel) Src was immunoprecipitated from the corresponding cell lysates shown in panel A with the Src GD11 antibody and its kinase activity determined in vitro using a Src peptide substrate with [γ-32P]ATP as previously done. Based on these results, all other transfection experiments were performed with 3-4 μg of DEP-1 plasmids to achieve optimal Src activation in these cells. (B) HEK 293T cells were transfected with empty vector (pmT2), WT DEP-1 and the indicated mutants, and the phosphorylation level of Src on Y529 and Y418 analyzed as described in panel A. (C) DEP-1 is constitutively tyrosine phosphorylated in HEK 293T cells. Total cell lysates of HEK 293T cells transfected with equal levels of WT DEP-1 and the various mutants were immunoblotted with a phospho-specific antibody detecting pY1320 DEP-1. (D) The catalytic activity of DEP-1 is not affected by the various mutations reducing its tyrosine phosphorylation. WT DEP-1 and mutants were coexpressed with activated Src Y527F in HEK 293T cells to enhance DEP-1 phosphorylation. DEP-1 was immunoprecipitated from 200 μg of HEK 293T cell lysates (not containing phosphatase inhibitors) and submitted to a pNPP hydrolysis assay. Empty vector (pmT2) and DEP-1 catalytically inactive C/S mutant represent negative controls. pNPP hydrolysis after 5 minutes of reaction is reported in this graph. (Bottom panel) Western blot analysis reveals similar levels of expressed WT DEP-1, mutants, and constitutively active Src in the various conditions tested; *P < .05. (E) Mutant DEP-1 is as effective as WT DEP-1 at dephosphorylating VEGFR2. HEK 293T cells cotransfected with VEGFR2 and either empty vector (pmT2), WT DEP-1, DEP-1 Y1311F/Y1320F (YY/FF), or the C/S mutant were stimulated with VEGF (50 ng/mL) for 5 minutes. Phosphorylation of immunoprecipitated VEGFR2 was detected using the general PY99 phosphotyrosine antibody (PY). Similar DEP-1 expression levels are shown. This result is representative of 2 independent experiments. All other results are representative of at least 3 independent experiments.
Mutation of Y1311 and Y1320 impairs DEP-1–mediated activation of Src in HEK 293T cells, without affecting PTP activity. (A) HEK 293T cells were transfected with increasing amounts of WT DEP-1 cDNA vector. The phosphorylation level of Src on Y529 and Y418 was determined by immunoblotting total cell lysates (TCL) with the corresponding phospho-specific antibodies. (Bottom panel) Src was immunoprecipitated from the corresponding cell lysates shown in panel A with the Src GD11 antibody and its kinase activity determined in vitro using a Src peptide substrate with [γ-32P]ATP as previously done. Based on these results, all other transfection experiments were performed with 3-4 μg of DEP-1 plasmids to achieve optimal Src activation in these cells. (B) HEK 293T cells were transfected with empty vector (pmT2), WT DEP-1 and the indicated mutants, and the phosphorylation level of Src on Y529 and Y418 analyzed as described in panel A. (C) DEP-1 is constitutively tyrosine phosphorylated in HEK 293T cells. Total cell lysates of HEK 293T cells transfected with equal levels of WT DEP-1 and the various mutants were immunoblotted with a phospho-specific antibody detecting pY1320 DEP-1. (D) The catalytic activity of DEP-1 is not affected by the various mutations reducing its tyrosine phosphorylation. WT DEP-1 and mutants were coexpressed with activated Src Y527F in HEK 293T cells to enhance DEP-1 phosphorylation. DEP-1 was immunoprecipitated from 200 μg of HEK 293T cell lysates (not containing phosphatase inhibitors) and submitted to a pNPP hydrolysis assay. Empty vector (pmT2) and DEP-1 catalytically inactive C/S mutant represent negative controls. pNPP hydrolysis after 5 minutes of reaction is reported in this graph. (Bottom panel) Western blot analysis reveals similar levels of expressed WT DEP-1, mutants, and constitutively active Src in the various conditions tested; *P < .05. (E) Mutant DEP-1 is as effective as WT DEP-1 at dephosphorylating VEGFR2. HEK 293T cells cotransfected with VEGFR2 and either empty vector (pmT2), WT DEP-1, DEP-1 Y1311F/Y1320F (YY/FF), or the C/S mutant were stimulated with VEGF (50 ng/mL) for 5 minutes. Phosphorylation of immunoprecipitated VEGFR2 was detected using the general PY99 phosphotyrosine antibody (PY). Similar DEP-1 expression levels are shown. This result is representative of 2 independent experiments. All other results are representative of at least 3 independent experiments.
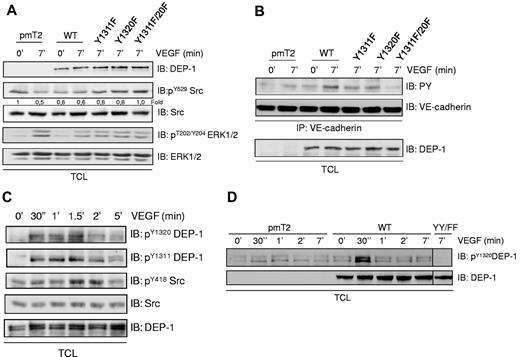
DEP-1 Y1311 and Y1320 are required for VEGF-dependent Src activation in endothelial cells
We previously demonstrated using an RNAi approach that DEP-1 is required for VEGF-induced Src activation in primary cultures of endothelial cells.15 Overexpression of DEP-1 reveals here that similarly to what was observed in HEK 293T cells (Figure 3A), moderate versus high expression levels of DEP-1 in endothelial cells also led to opposite effects on Src activation and phosphorylation of its substrate VE-cadherin (supplemental Figure 2A).37,38 This is consistent with the notion that higher expression of endogenous DEP-1 is observed in postconfluent or quiescent endothelial cells in vitro and in vivo, while its expression is decreased to moderate levels in growing and migrating cells.3,13 To find out whether Src activation also relied on DEP-1 Y1311 and Y1320 in VEGF-stimulated endothelial cells, we therefore investigated the consequences of expressing moderate amounts of WT and DEP-1 Y/F mutants in primary cultures of bovine aortic endothelial cells (BAECs). Figure 4A shows that VEGF stimulation of control cells (pmT2-transfected) led to the dephosphorylation of Src Y529, while this was observed constitutively in cells overexpressing WT DEP-1. In contrast, a progressive decrease in Y529 dephosphorylation induced by VEGF was observed when Y1311F, Y1320F, and Y1311F/Y1320F mutants were expressed, the latter being the most efficient at inhibiting Src Y529 dephosphorylation. In these same conditions, the VEGF-induced phosphorylation of ERK1/2 was similarly decreased in cells overexpressing WT DEP-1 or the mutants, demonstrating that impaired dephosphorylation of Src in cells expressing the DEP-1 mutants was not because of differential VEGFR2-dependent signaling or to reduced phosphatase activity. Our results also demonstrated that the Y1311F and Y1320F mutants but even more strikingly the double Y1311F/Y1320F mutant abolished the Src-dependent phosphorylation of VE-cadherin in response to VEGF stimulation, confirming the role of DEP-1 Y1311/Y1320 in VEGF-dependent Src activation (Figure 4B). To strengthen this observation, the sequence downstream of Y1320 (Y1320ENL), corresponding to a potent Src SH2 binding site, was mutated to Y1320QNL (supplemental Figure 3). Results show that the association of Src with the DEP-1 C/S E1321Q mutant was much reduced and correlated with the impaired ability of VEGF to induce the dephosphorylation of Src Y529 and the phosphorylation of VE-cadherin in endothelial cells expressing this mutant. These experiments thus reveal the requirement of DEP-1 Y1311 and Y1320 for Src association and activation in VEGF-stimulated endothelial cells.
VEGF-dependent phosphorylation of DEP-1 on Y1311 and Y1320 mediate Src activation in endothelial cells. (A) BAECS were transfected with empty vector (pmT2), WT DEP-1, and the indicated mutants, serum-starved, and then stimulated with VEGF (50 ng/mL) for 7 minutes. Src dephosphorylation on Y529 was detected using a phospho-specific antibody recognizing phosphorylated Y529. Densitometry ratios of pY529Src/Src levels were quantified using Quantity One software from Bio-Rad. As an indication of VEGF-dependent signaling, the activation of ERK1/2 was monitored by detecting phosphorylated T202/Y204. Equivalent signaling is observed in cells expressing WT DEP-1 and mutants. (B) BAECs were treated as in panel A. The phosphorylation of VE-cadherin was investigated after the immunoprecipitation of VE-cadherin and its immunodetection with the PY99 antibody. (C) HUVECs were serum-starved and stimulated with VEGF for the indicated times. Phosphorylation of endogenous DEP-1 is detected in total cell lysates (TCL) using phospho-specific antibodies detecting pY1311 and pY1320. The phosphorylation/activation status of Src on Y418 is determined by immunoblotting total cell lysates with the pY418Src antibody. Results suggest that VEGF-induced DEP-1 tyrosine phosphorylation is concomitant with Src activation. (D) The VEGF-mediated tyrosine phosphorylation of DEP-1 on Y1320 is enhanced in BAECs overexpressing WT DEP-1, but abrogated in cells expressing the Y1311F/Y1320F mutant (YY/FF). These results are representative of at least 3 independent experiments.
VEGF-dependent phosphorylation of DEP-1 on Y1311 and Y1320 mediate Src activation in endothelial cells. (A) BAECS were transfected with empty vector (pmT2), WT DEP-1, and the indicated mutants, serum-starved, and then stimulated with VEGF (50 ng/mL) for 7 minutes. Src dephosphorylation on Y529 was detected using a phospho-specific antibody recognizing phosphorylated Y529. Densitometry ratios of pY529Src/Src levels were quantified using Quantity One software from Bio-Rad. As an indication of VEGF-dependent signaling, the activation of ERK1/2 was monitored by detecting phosphorylated T202/Y204. Equivalent signaling is observed in cells expressing WT DEP-1 and mutants. (B) BAECs were treated as in panel A. The phosphorylation of VE-cadherin was investigated after the immunoprecipitation of VE-cadherin and its immunodetection with the PY99 antibody. (C) HUVECs were serum-starved and stimulated with VEGF for the indicated times. Phosphorylation of endogenous DEP-1 is detected in total cell lysates (TCL) using phospho-specific antibodies detecting pY1311 and pY1320. The phosphorylation/activation status of Src on Y418 is determined by immunoblotting total cell lysates with the pY418Src antibody. Results suggest that VEGF-induced DEP-1 tyrosine phosphorylation is concomitant with Src activation. (D) The VEGF-mediated tyrosine phosphorylation of DEP-1 on Y1320 is enhanced in BAECs overexpressing WT DEP-1, but abrogated in cells expressing the Y1311F/Y1320F mutant (YY/FF). These results are representative of at least 3 independent experiments.
We next investigated whether endogenous DEP-1 was phosphorylated in response to VEGF using DEP-1 phospho-specific antibodies. Figure 4C shows that phosphorylation of DEP-1 on Y1311 and Y1320 was rapid and transient, with maximal increases occurring within 2 minutes. A concurrent increase in Src Y418 phosphorylation was also observed over this time period. Importantly, similar kinetics of DEP-1 phosphorylation on Y1320 were observed in VEGF-stimulated BAECs overexpressing WT DEP-1, but phosphorylation was virtually absent in VEGF-stimulated cells overexpressing the Y1311F/Y1320F mutant where Src activation was found to be abrogated (Figure 4D). These results are thus consistent with our model hypothesis that tyrosine phosphorylation of DEP-1 is part of the mechanism leading to Src activation, and that a phospho-displacement mechanism may be involved.
DEP-1 promotes the VEGF-dependent remodeling of intercellular junctions and endothelial permeability via Y1311 and Y1320
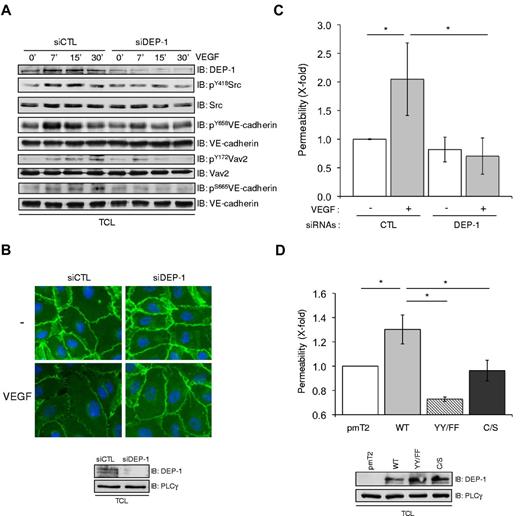
VEGF-dependent remodeling and loosening of intercellular adhesions are required not only to facilitate tip cell migration and invasion during sprouting angiogenesis,27 but also represent key steps in the promotion of vascular permeability.25 Src is a major regulator of these processes, in part through the direct tyrosine phosphorylation of adhesion proteins including VE-cadherin, and also via the induction of a Src-VAV2-Rac-PAK pathway, resulting in the serine phosphorylation of VE-cadherin and its cellular internalization.24,37-40 Consistent with decreased Src Y418 phosphorylation, Figure 5A shows that the VEGF-induced tyrosine phosphorylation of VE-cadherin, as detected with the pY658VE-cadherin antibody, was reduced in DEP-1–depleted cells. In addition, the Src-dependent phosphorylation/activation of the Rac GDP exchange factor VAV2 was similarly decreased and correlated with the inhibited phosphorylation of VE-cadherin on serine 665 (antibody specificity in supplemental Figure 4). As phosphorylation of VE-cadherin correlates with its increased internalization and the weakening of its intracellular association with catenins at cell-cell junctions,38,39 the above results suggested that the remodeling of cell-cell junctions and endothelial permeability might be defective in cells lacking DEP-1 expression. Accordingly, immunostaining of HUVECs with a β-catenin antibody revealed a “hairy” phenotype and the appearance of intercellular gaps representative of loosened cell-cell contacts on stimulation of control cells with VEGF, while DEP-1–silenced cells maintained stable cell-cell junctions (Figure 5B). In agreement with this, HUVECs transfected with control siRNAs cells showed a significant increase in permeability in response to VEGF, while this was completely blocked in DEP-1–depleted cell monolayers (Figure 5C). Importantly, overexpression of WT DEP-1 in HUVECs promoted VEGF-dependent endothelial cell permeability compared with cells transfected with empty vector (pmT2; Figure 5D). However, overexpression of the Y1311F/Y1320F mutant decreased permeability below the level detected in control cells, thus behaving as a dominant-negative mutant. These results are consistent with a role for DEP-1 and its phosphorylated C-terminal tail in Src activation and the consequent phosphorylation of VE-cadherin in VEGF-stimulated endothelial cells, and highlight the role of DEP-1 in the control of endothelial permeability.
DEP-1 and its C-terminal Y1311 and Y1320 are essential mediators of VEGF-dependent endothelial permeability. (A) DEP-1 promotes VEGF-dependent VE-cadherin phosphorylation. HUVECs were transfected with control (CTL) or DEP-1 siRNAs, serum-starved for 6 hours, and then stimulated with VEGF for the indicated times. The phosphorylation level of Src, VE-cadherin, and Vav2 was determined by immunoblotting with the indicated phospho-specific antibodies. Results are representative of at least 3 independent experiments. (B) DEP-1 is required for VEGF-induced loosening of cell-cell junctions. Control (CTL) and DEP-1–depleted cells were stimulated with VEGF (50 ng/mL) for 30 minutes and stained with β-catenin and anti–mouse Alexa 488 secondary antibodies. Bottom panel, RNAi-mediated decrease in DEP-1 expression levels. Results are representative of 3 independent experiments. (C) DEP-1 mediates VEGF-induced endothelial permeability. Control (CTL) and DEP-1–silenced cells were plated on collagen-coated inserts. Forty-six hours later, cells were serum-starved for 2 hours and then stimulated or not with VEGF (50 ng/mL) for 30 minutes in the presence of FITC-dextran (in the upper chamber). VEGF-induced permeability was measured by detecting the fluorescence emitted by FITC-dextran present in aliquots that were collected from the bottom chambers. Data are presented as fold changes over unstimulated control (CTL) cells. Assays were conducted in triplicates and results are representative of 4 independent experiments; *P < .05. (D) HUVECs transfected with pmT2 empty vector, WT DEP-1, the Y1311F/1320F (YY/FF), or C/S mutants were plated on collagen-coated inserts, serum-starved for 1 hour and then stimulated for 30 minutes with VEGF in the presence of FITC-dextran, as described in panel C. Data are presented as fold changes over stimulated control (pmT2) cells. Assays were conducted in triplicates and results are representative of 3 independent experiments.
DEP-1 and its C-terminal Y1311 and Y1320 are essential mediators of VEGF-dependent endothelial permeability. (A) DEP-1 promotes VEGF-dependent VE-cadherin phosphorylation. HUVECs were transfected with control (CTL) or DEP-1 siRNAs, serum-starved for 6 hours, and then stimulated with VEGF for the indicated times. The phosphorylation level of Src, VE-cadherin, and Vav2 was determined by immunoblotting with the indicated phospho-specific antibodies. Results are representative of at least 3 independent experiments. (B) DEP-1 is required for VEGF-induced loosening of cell-cell junctions. Control (CTL) and DEP-1–depleted cells were stimulated with VEGF (50 ng/mL) for 30 minutes and stained with β-catenin and anti–mouse Alexa 488 secondary antibodies. Bottom panel, RNAi-mediated decrease in DEP-1 expression levels. Results are representative of 3 independent experiments. (C) DEP-1 mediates VEGF-induced endothelial permeability. Control (CTL) and DEP-1–silenced cells were plated on collagen-coated inserts. Forty-six hours later, cells were serum-starved for 2 hours and then stimulated or not with VEGF (50 ng/mL) for 30 minutes in the presence of FITC-dextran (in the upper chamber). VEGF-induced permeability was measured by detecting the fluorescence emitted by FITC-dextran present in aliquots that were collected from the bottom chambers. Data are presented as fold changes over unstimulated control (CTL) cells. Assays were conducted in triplicates and results are representative of 4 independent experiments; *P < .05. (D) HUVECs transfected with pmT2 empty vector, WT DEP-1, the Y1311F/1320F (YY/FF), or C/S mutants were plated on collagen-coated inserts, serum-starved for 1 hour and then stimulated for 30 minutes with VEGF in the presence of FITC-dextran, as described in panel C. Data are presented as fold changes over stimulated control (pmT2) cells. Assays were conducted in triplicates and results are representative of 3 independent experiments.
Efficient endothelial cell invasion and capillary formation require DEP-1 and its phosphorylated C-terminal tail
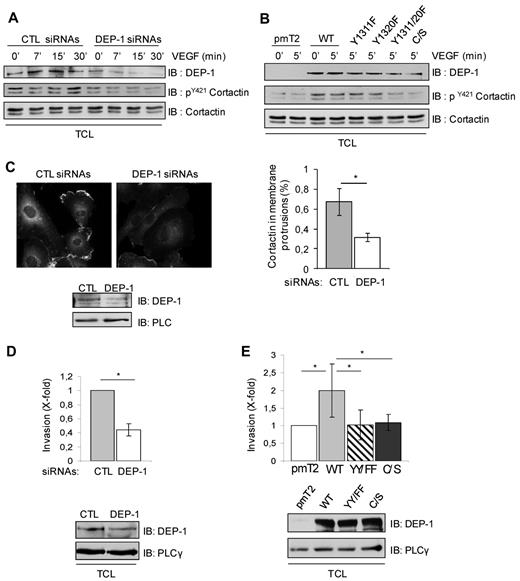
Src is also a key promoter of endothelial cell invasion and neovascularization.24,33 One potential candidate substrate mediating these effects is Cortactin, which allows branched actin polymerization and the stabilization of membrane protrusions during cell migration and invasion.41 It is enriched in membrane protrusions of endothelial cells in a Src-dependent manner and is essential for VEGF-induced migration and in vivo angiogenesis.42-44 Importantly, Cortactin tyrosine phosphorylation also correlates with its ability to promote cell migration and invasion.41,44,45 Consistent with the role of DEP-1 via Y1311/Y1320 in the activation of Src, the depletion of DEP-1 in HUVECs or the expression of the DEP-1 Y1311F/Y1320F mutant in BAECs resulted in the decreased tyrosine phosphorylation of Cortactin, as detected using a pY421Cortactin antibody (Figure 6A-B). In contrast, Cortactin phosphorylation was stimulated by overexpression of WT DEP-1 (Figure 6B). As previously reported,43 Figure 6C shows that Cortactin was enriched in the membrane protrusions of control endothelial cells stimulated with VEGF, but this localization was impaired in the DEP-1–depleted HUVEC population. In agreement with these observations, the VEGF-dependent invasion of DEP-1–silenced HUVECs into Matrigel was strongly impaired (Figure 6D). Conversely, overexpression of WT DEP-1 stimulated this response over control cells (pmT2-transfected), while expression of the Y1311F/Y1320F or C/S mutants were unable to do so (Figure 6E). These results thus show that DEP-1 via Y1311/Y1320 is required for the proper tyrosine phosphorylation and localization of Cortactin to membrane protrusions, and is an essential mediator of endothelial cell invasion triggered by VEGF. It is interesting to note here that VEGF-stimulated endothelial cells expressing high levels of DEP-1, which rather mimic the status of quiescent/postconfluent endothelial cells,3,13 were unable to invade compared with pmT2-transfected cells or cells expressing moderate amounts of DEP-1 (supplemental Figure 2B). These results are thus consistent with the opposite capacity of moderate versus high levels of DEP-1 to activate Src.
DEP-1 promotes VEGF-induced Cortactin tyrosine phosphorylation and endothelial cell invasion through Y1311 and Y1320. (A) HUVECs were transfected with control (CTL) and DEP-1 siRNAs and then stimulated with VEGF (50 ng/mL) as indicated in legend to Figure 5A. Cortactin tyrosine phosphorylation was monitored by immunoblotting total cell lysates (TCL) with the pY421Cortactin antibody. DEP-1 expression and Cortactin protein levels were detected with the DEP-1 goat and Cortactin (4F11clone) antibodies, respectively. (B) BAECs were transfected with WT and mutant DEP-1 constructs, serum-starved, and then stimulated 5 minutes with VEGF (50 ng/mL). Cortactin tyrosine phosphorylation and protein levels were monitored as described in panel A. (C) DEP-1 is required for the localization of Cortactin in membrane protrusions. HUVECs transfected with control (CTL) or DEP-1siRNAs were plated on gelatin-coated glass coverslips, serum-starved, and then stimulated with VEGF (10 ng/mL) for 5 minutes before cell fixation. Immunostaining with Cortactin (4F11 clone) and Alexa Fluor 594–coupled mouse secondary antibodies is shown. The percentage of cells with enriched localization of Cortactin in membrane protrusions was evaluated in control and DEP-1–depleted cells in 4 microscopic fields at ×40 magnification; *P < .05. (D) HUVECs were transfected with control (CTL) or DEP-1 siRNAs and seeded in duplicates 48 hours after transfection on Transwell filter inserts previously coated with 50 μL of Matrigel (2 mg/mL). Cells were allowed to invade for 24 hours, and then processed to visualize and count cells on the lower side of the filter. Results ± SD have been normalized using the average from the control condition; *P < .05. (E) HUVECs transfected with empty vector (pmT2), WT DEP-1, DEP-1 Y1311F/Y1320F (YY/FF), or DEP-1 C/S were plated in duplicates on Matrigel-coated filter inserts and processed as described in panel D. Results are representative of 5 independent experiments; *P < .05. All other results shown in this figure are representative of 3 independent experiments.
DEP-1 promotes VEGF-induced Cortactin tyrosine phosphorylation and endothelial cell invasion through Y1311 and Y1320. (A) HUVECs were transfected with control (CTL) and DEP-1 siRNAs and then stimulated with VEGF (50 ng/mL) as indicated in legend to Figure 5A. Cortactin tyrosine phosphorylation was monitored by immunoblotting total cell lysates (TCL) with the pY421Cortactin antibody. DEP-1 expression and Cortactin protein levels were detected with the DEP-1 goat and Cortactin (4F11clone) antibodies, respectively. (B) BAECs were transfected with WT and mutant DEP-1 constructs, serum-starved, and then stimulated 5 minutes with VEGF (50 ng/mL). Cortactin tyrosine phosphorylation and protein levels were monitored as described in panel A. (C) DEP-1 is required for the localization of Cortactin in membrane protrusions. HUVECs transfected with control (CTL) or DEP-1siRNAs were plated on gelatin-coated glass coverslips, serum-starved, and then stimulated with VEGF (10 ng/mL) for 5 minutes before cell fixation. Immunostaining with Cortactin (4F11 clone) and Alexa Fluor 594–coupled mouse secondary antibodies is shown. The percentage of cells with enriched localization of Cortactin in membrane protrusions was evaluated in control and DEP-1–depleted cells in 4 microscopic fields at ×40 magnification; *P < .05. (D) HUVECs were transfected with control (CTL) or DEP-1 siRNAs and seeded in duplicates 48 hours after transfection on Transwell filter inserts previously coated with 50 μL of Matrigel (2 mg/mL). Cells were allowed to invade for 24 hours, and then processed to visualize and count cells on the lower side of the filter. Results ± SD have been normalized using the average from the control condition; *P < .05. (E) HUVECs transfected with empty vector (pmT2), WT DEP-1, DEP-1 Y1311F/Y1320F (YY/FF), or DEP-1 C/S were plated in duplicates on Matrigel-coated filter inserts and processed as described in panel D. Results are representative of 5 independent experiments; *P < .05. All other results shown in this figure are representative of 3 independent experiments.
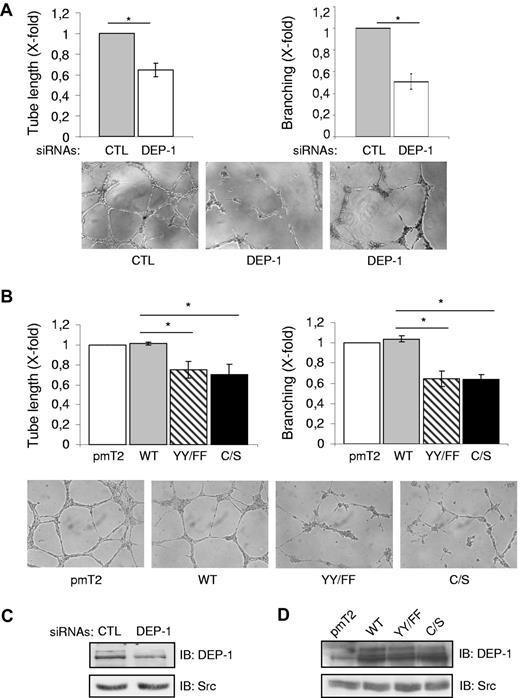
As could be expected based on the inhibition of Src and Cortactin phosphorylation, the plating of DEP-1–silenced HUVECs on Matrigel resulted in their impaired ability to reorganize and elongate branched capillary-like structures (Figure 7A). Expression of the DEP-1 Y1311F/Y1320F or C/S mutants also reduced the length of capillaries forming and significantly impaired branching compared with cells overexpressing WT DEP-1 (Figure 7B). Of note, there was no difference in the capacity of DEP-1–silenced cells or cells expressing the DEP-1 Y1311F/Y1320F mutant to adhere to Matrigel with that of control cells, demonstrating that the impaired invasion and capillary formation observed were not a consequence of decreased adhesion (supplemental Figure 5). Altogether, the results presented herein demonstrate for the first time the central implication of endogenously expressed DEP-1 in the mediation of Src-dependent angiogenic responses and reveal the essential contribution of DEP-1 Y1311 and Y1320 in the proper regulation of these events.
DEP-1, via Y1311 and Y1320, mediates the formation of branching capillary-like structures. (A) HUVECs transfected with control (CTL) or DEP-1 siRNAs were plated 48 hours after transfection on Matrigel (in duplicates) to promote the formation of capillary-like structures. The length of tubes formed as well as the number of branches at connecting nodes were quantified after 5-6 hours from 6 fields at ×10 magnification /well. Results ± SD have been normalized using the average from the control condition and are representative of 3 independent experiments; *P < .05. (B) HUVECS transfected with the indicated constructs were plated in duplicates on Matrigel to evaluate their ability to form branching capillary-like structures. Quantification was done as described in panel A and is representative of 3 independent experiments; *P < .05. (C) Representative DEP-1 expression level in RNAi-transfected cells. (D) Comparable expression level of DEP-1 and mutants in transfected HUVECs.
DEP-1, via Y1311 and Y1320, mediates the formation of branching capillary-like structures. (A) HUVECs transfected with control (CTL) or DEP-1 siRNAs were plated 48 hours after transfection on Matrigel (in duplicates) to promote the formation of capillary-like structures. The length of tubes formed as well as the number of branches at connecting nodes were quantified after 5-6 hours from 6 fields at ×10 magnification /well. Results ± SD have been normalized using the average from the control condition and are representative of 3 independent experiments; *P < .05. (B) HUVECS transfected with the indicated constructs were plated in duplicates on Matrigel to evaluate their ability to form branching capillary-like structures. Quantification was done as described in panel A and is representative of 3 independent experiments; *P < .05. (C) Representative DEP-1 expression level in RNAi-transfected cells. (D) Comparable expression level of DEP-1 and mutants in transfected HUVECs.
Discussion
Previous studies from our laboratory demonstrated that the knockdown of DEP-1 expression in endothelial cells impaired Src activation in response to VEGF and fibroblast growth factor (FGF).15 We further showed that this was a consequence of the defective dephosphorylation of the Src inhibitory Y529 in the VE-cadherin–associated fraction, which was identified as the major pool of activated Src in VEGF-stimulated endothelial cells.46 We now show that the VEGF-induced activation of Src is mediated through the phosphorylation of DEP-1 on the C-terminal Y1311 and Y1320, which allow its interaction with the Src SH2 domain and the dephosphorylation of the displaced inhibitory Y529. Consistent with the central role played by Src in angiogenesis, our studies further reveal the major function of DEP-1 and its C terminus in the VEGF-dependent phosphorylation of VE-cadherin and Cortactin,42,47 and consequently, in the remodeling of intercellular junctions, permeability, cell invasion, and the formation of branching capillary-like structures. We thus propose that a phospho-displacement mechanism engaging DEP-1 pY1311 and pY1320 with the SH2 domain of Src is required for its activation in response to VEGF stimulation, and that this is critical for the angiogenic response of endothelial cells.
The impaired capacity of DEP-1 Y/F mutants to associate with Src and promote its activation (Figures 2,Figure 3–4) reveals the importance of VEGF-dependent phosphorylation of DEP-1 as a mechanism controlling Src activation in this cell system. Supporting this conclusion, we found that the phosphorylation kinetics of DEP-1 overlapped with those of VEGF-induced Src Y418 phosphorylation (Figure 4). In addition, while phosphorylation of overexpressed WT DEP-1 on Y1320 was above that seen in control cells on VEGF stimulation, no residual phosphorylation was detected in cells overexpressing the DEP-1 Y1311F/Y1320F mutant where Src activation was abrogated (Figure 4). On these bases, we then propose that the VEGF-dependent phosphorylation of Y1311/Y1320 controls the moment at which DEP-1 can interact with Src to dephosphorylate Y529, but without altering its ability to dephosphorylate other substrates not associating with DEP-1 via these residues. This mechanism is somewhat similar to what was reported for PTPϵ, whereby tyrosine phosphorylation of the C-terminal Y695 is essential for the specific dephosphorylation of Src on Y529, but not for the ability of PTPϵ to dephosphorylate other substrates.31 Importantly, we also demonstrated that the impaired ability of mutant DEP-1 to dephosphorylate Src was not because of its decreased catalytic activity, because it was as active as WT DEP-1 in a pNPP assay or after its coexpression with another substrate, VEGFR2 (Figure 3). Our results therefore identify growth factor-mediated phosphorylation of DEP-1 as another level of regulation of its functions, by determining both substrate specificity and kinetics of dephosphorylation.
We have shown in HEK 293T cells that DEP-1 is constitutively phosphorylated, and that this is dependent on the basal activity of SFKs (Figures 1 and 3C). However, it is not clear yet how VEGF leads to this initial phosphorylation of DEP-1 in endothelial cells. As we have concluded from our work in HEK 293T cells, DEP-1 can auto-dephosphorylate or get dephosphorylated by other PTPs (Figure 1). Thus, it is tempting to speculate that a VEGF-induced event leading to the attenuation of PTP catalytic activity could contribute to the increased phosphorylation of DEP-1. In that respect, reactive oxygen species (ROS), which have inhibitory effects on PTPs, might perhaps be involved.36 Coupled with a relatively high basal activity of Src in endothelial cells, the VEGF-induced increase in ROS40 could lead to a decrease in DEP-1 activity, thus enabling the accumulation of phosphorylated DEP-1 and the consequent activation of Src. In agreement with this idea, it is interesting that ROS production in endothelial cells contributes to VEGF-induced Src activation and phosphorylation of VE-cadherin, and to the promotion of capillary formation and permeability.40,48
To further prove the contribution of DEP-1 Y1311/Y1320 to VEGF-mediated Src activation, we demonstrated that the phosphorylation of 2 well-known Src substrates, VE-cadherin and Cortactin,38,41 was abrogated in cells expressing the Y1311F/Y1320F, E1321Q or C/S mutants, similarly to what was observed in DEP-1–silenced cells (Figures 4,Figure 5–6, supplemental Figure 3). VE-cadherin tyrosine and serine phosphorylation is associated with the loosening of cell-cell junctions that promotes increased vessel permeability and the initiation of vessel sprouting and elongation.25,27 In the case of Cortactin, its tyrosine phosphorylation promotes the local polymerization of branched actin that is important for stabilization of nascent membrane protrusions during cell migration and invasion.41,45,49 Its expression and tyrosine phosphorylation are also essential for VEGF-dependent migration.42,44 In this context, the decreased phosphorylation of VE-cadherin and Cortactin, as well as the impaired localization of Cortactin at membrane protrusions, is highly consistent with the inability of DEP-1–silenced or mutant-expressing cells to remodel cell-cell junctions and regulate permeability in response to VEGF, or to invade and form a well-organized network of branching capillary-like structures (Figures 5,Figure 6–7). These data thus demonstrate for the first time a promoting role for endogenous and moderately overexpressed DEP-1 in the induction of proangiogenic functions of endothelial cells and also highlight the key role played by the C-terminal Y1311/Y1320. These findings also suggest that the defective vessel remodeling and branching characterized in the DEP-1 mutant mouse23 might partly be explained by impaired Src-dependent pathways. The reported localization of DEP-1 at endothelial cell-cell junctions22 and its association with constituents of the VE-cadherin complex11,14,15 strongly support its critical role in the activation of Src at this site46 (Figures 4B, 5A). However, as DEP-1 also promotes cell-substratum adhesions,12 its possible involvement to these sites as well is not excluded.
The results presented herein also importantly revealed that the expression level of DEP-1 is a critical factor dictating its substrate specificity and function. Moderate overexpression of DEP-1 increased Src activation associated with Y529 dephosphorylation, while higher levels also led to Y418 dephosphorylation and blocked VEGF-dependent tyrosine phosphorylation of VE-cadherin and endothelial cell invasion (Figures 3–4, supplemental Figure 2). Thus, depending on DEP-1 expression levels, the dephosphorylation of Src might be inappropriate for the promotion of biologic activities such as permeability and invasion. In that context, it is interesting to consider that similarly to the in vitro situation, the expression of DEP-1 in vivo is decreased in proliferating and migrating cells during vessel repair compared with adjacent quiescent endothelial cells.3,13 This then suggests that DEP-1 might have bivalent functions, where moderate levels would promote VEGF-dependent Src activation in actively growing and invading endothelial cells, while its up-regulated expression at confluence and the recruitment of VEGFR221 would result in the down-regulation of both Src and VEGFR2 activity13,15 to promote vessel quiescence and stabilization. Interestingly, a similar dual function of the receptor-like PTP CD45 was previously reported, where higher versus lower expression levels led to the differential dephosphorylation and activation of the SFK Lck, inducing both positive and negative regulatory functions in T-cell signaling.50 In all cases, these regulatory mechanisms might be necessary to allow the down-regulation of SFK signaling and the attenuation/termination of biologic responses associated with cell quiescence.
In conclusion, these studies bring novel insights into the molecular mechanisms underlying the VEGF-dependent regulation of Src, which is a key promoter of angiogenesis and vascular permeability, and importantly recognize DEP-1 Y1311 and Y1320 as positive regulators of the angiogenic response.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Nicholas Tonks, Jeroen den Hertog, Joan Brugge, Stéphane Laporte, and Filippo Giancotti (via Addgene) for the various plasmids used in this study; André Veillette for the Fyn antibody; and Marie-Josée Hébert for providing the SYF MEF cell line. They also thank Marie-Claude Gingras (Michel Tremblay's laboratory) for help with the pNPP assay, and Anne-Marie Mes-Masson and Nathalie Grandvaux for their comments on the manuscript.
This work was mainly supported by the Cancer Research Society Inc (I.R.), and was completed with funds from the Canadian Institutes of Health Research (MOP-93681; I.R.).
Authorship
Contribution: K.S., C. Chabot, S.L., L.L. and C. Caron performed the experiments; K.S., C. Chabot, and S.L. contributed to discussions on the design of experiments and interpretation of the data with I.R; N.T.N.T. made the Myr-DEP-1 Y/F mutants; J.K.H. performed experiments in supplemental Figure 4; J.G. contributed reagent and protocol; M.E. participated in discussions and contributed reagents; I.R. designed the research project and analyzed the data; and I.R. wrote the manuscript with the contributions of K.S. and C. Chabot.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Isabelle Royal, CRCHUM, Hôpital Notre-Dame, 1560 rue Sherbrooke est, Montréal, QC, Canada, H2L 4M1; e-mail: isabelle.royal@umontreal.ca.

![Figure 3. Mutation of Y1311 and Y1320 impairs DEP-1–mediated activation of Src in HEK 293T cells, without affecting PTP activity. (A) HEK 293T cells were transfected with increasing amounts of WT DEP-1 cDNA vector. The phosphorylation level of Src on Y529 and Y418 was determined by immunoblotting total cell lysates (TCL) with the corresponding phospho-specific antibodies. (Bottom panel) Src was immunoprecipitated from the corresponding cell lysates shown in panel A with the Src GD11 antibody and its kinase activity determined in vitro using a Src peptide substrate with [γ-32P]ATP as previously done. Based on these results, all other transfection experiments were performed with 3-4 μg of DEP-1 plasmids to achieve optimal Src activation in these cells. (B) HEK 293T cells were transfected with empty vector (pmT2), WT DEP-1 and the indicated mutants, and the phosphorylation level of Src on Y529 and Y418 analyzed as described in panel A. (C) DEP-1 is constitutively tyrosine phosphorylated in HEK 293T cells. Total cell lysates of HEK 293T cells transfected with equal levels of WT DEP-1 and the various mutants were immunoblotted with a phospho-specific antibody detecting pY1320 DEP-1. (D) The catalytic activity of DEP-1 is not affected by the various mutations reducing its tyrosine phosphorylation. WT DEP-1 and mutants were coexpressed with activated Src Y527F in HEK 293T cells to enhance DEP-1 phosphorylation. DEP-1 was immunoprecipitated from 200 μg of HEK 293T cell lysates (not containing phosphatase inhibitors) and submitted to a pNPP hydrolysis assay. Empty vector (pmT2) and DEP-1 catalytically inactive C/S mutant represent negative controls. pNPP hydrolysis after 5 minutes of reaction is reported in this graph. (Bottom panel) Western blot analysis reveals similar levels of expressed WT DEP-1, mutants, and constitutively active Src in the various conditions tested; *P < .05. (E) Mutant DEP-1 is as effective as WT DEP-1 at dephosphorylating VEGFR2. HEK 293T cells cotransfected with VEGFR2 and either empty vector (pmT2), WT DEP-1, DEP-1 Y1311F/Y1320F (YY/FF), or the C/S mutant were stimulated with VEGF (50 ng/mL) for 5 minutes. Phosphorylation of immunoprecipitated VEGFR2 was detected using the general PY99 phosphotyrosine antibody (PY). Similar DEP-1 expression levels are shown. This result is representative of 2 independent experiments. All other results are representative of at least 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/13/10.1182_blood-2011-12-398040/4/m_zh89991297130003.jpeg?Expires=1769294954&Signature=IRH1PoXgFb~XCirJ4QCw4JYFkaSC7Ag66n2X0~58yo8kgmKRi5XTZDYBxD6dAjUN6SaAtWkkvImW-lTyhD8cmzSmLxXMpoOJFbuZ0zRJEtXsMfw1AMpK5fhpjwQkkv-8DuoGu7gAxIlTOpB3~Tbxa2fhMoluc-6BwHUZwcGt8aDXprW0GICPKyH8y8LB5Da4rrFGgKlyrHOSdGsuekaxP0X5PcvA10i6kR9ATrAL1MXxLQiMNoYXlFAUpwtVe-18r-KaZwpqAFOTPxv94oxXxCGNemVWRD4xFKVs1--DFeBzuDLvY6y9Q~UNJFB2eBWQXX48f8BlSoD9nmZVQFv9Bg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)