Abstract
Successful differentiation and expansion of endothelial cells (ECs) from embryonic stem cell (ESC)–derived Flk1+ mesodermal precursor cells (MPCs) requires supplementation of vascular endothelial growth factor-A (VEGF-A). While analyzing VEGF-A/VEGFR2 downstream signaling pathway that underlies the VEGF-A-induced differentiation and expansion of ECs, we fortuitously found that Rho-associated protein kinase (ROCK) inhibitor Y27632 profoundly promoted the differentiation and expansion of ECs from Flk1+ MPCs while reducing the differentiation and expansion of mural cells. The ROCK suppression-induced expansion of ECs appears to have resulted from promotion of proliferation of ECs via activation of PI3-kinase-Akt signaling. The ECs obtained by the combination of ROCK suppression and VEGF-A supplementation faithfully expressed most pan-EC surface makers, and phenotypic analyses revealed that they were differentiated toward arterial EC. Further incubation of the ICAM2+ ECs with Y27632 and VEGF-A for 2 days promoted expansion of ECs by 6.5-fold compared with those incubated with only VEGF-A. Importantly, the ROCK suppression-induced ECs displayed neovasculogenic abilities in vitro and in vivo. Thus, supplementation of ROCK inhibitor Y27632 along with VEGF-A in 2D Matrigel culture system provides a simple, efficient, and versatile method for obtaining ample amount of ESC-derived ECs at high purity suitable for use in therapeutic neovascularization.
Introduction
Therapeutic neovascularization using endothelial cells (ECs) derived from embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) holds great promise for patients with various kinds of ischemic diseases.1 To gain therapeutic competence, the ECs need to be of ample quantity as well as high purity after differentiation and purification process in vitro. Many attempts were made during the last decade to achieve this goal: research groups led by Nishikawa and Yamashita have each progressively established efficient systems for promoting the differentiation, specification, and expansion of ECs using ESC- and iPSC-derived Flk1+ mesodermal precursor cells (MPCs)1-6 ; by adapting these culture systems, researchers have found that differentiation, specification, and expansion of ECs from Flk1+ MPCs can be promoted by activation of cAMP/protein kinase A signaling,7-9 Ras-ERK signaling,10 PI3-kinase/Akt/PKC signaling,11 angiopoietin-1/Tie2 signaling,12 and suppression of TGF-β receptor kinase signaling,13,14 as well as implementation of a more favorable and adaptable growth medium or microenvironment.15,16 Last but not least, researchers have identified VEGF-A as a crucial and potent factor for differentiation of ECs; studies have shown that supplementation of vascular endothelial growth factor-A (VEGF-A) along with use of a defined medium eliminates the need for feeder cell coculture, thereby minimizing the level of cell contamination and further enhancing the purity of ECs.4,6,13
Because supplemental VEGF-A has been found to be the most essential component for differentiation and maintenance of ECs in a feeder cell-free microenvironment, we have investigated how VEGF-A regulates the differentiation of ECs. During the dissection of VEGF-A/VEGFR2 (KDR/Flk1) downstream signaling that underlies the VEGF-A–induced differentiation and expansion of ECs,17,18 we noticed that a small chemical compound Y27632, an inhibitor of Rho-associated protein kinase (ROCK), profoundly promotes the differentiation and expansion of ECs. ROCK is a major downstream effector protein of RhoA, which is one of Rho family small GTPases that control a diverse array of cellular processes, including cytoskeletal dynamics, cell polarity, membrane transport, and gene expression.19-21 ROCK consists of 2 subtypes, ROCK1 and ROCK2, which both carry critical functions in the regulation of actin cytoskeleton assembly across many cell types through direct activation of myosin light chain and inactivation of myosin phosphatase.19-22 ROCK also plays a key role in myogenic differentiation,23-25 and recent studies have shown that Y27632 blocks the dissociation-induced apoptosis in human ESCs through suppression of Rho-ROCK pathway-mediated hyperactivation of actomyosin.26-28
In the present study, we demonstrate how suppression of ROCK profoundly promotes the differentiation and expansion of ECs in our established feeder cell-free, 2-dimensional Matrigel system. Moreover, we show how ROCK suppression could be applied in obtaining ECs from ESCs at a sufficiently high quantity and purity suitable for therapeutic purpose.
Methods
Cell culture and reagents
E14tg2a ESC cell line was a generous gift from Dr Jun K. Yamashita (Kyoto University), and R1 ESC cell line was purchased from ATCC. Mouse iPSC derived from FVB strain was a generous gift from Drs Hyun-Jai Cho and Hyo-Soo Kim (Seoul National University Hospital).29 To obtain Flk1+ MPCs, ESCs and iPSCs were cultured on 0.1% gelatin-coated plates at a density of 1-2 × 103 cells/cm2 in differentiation medium (α-minimal essential medium, Invitrogen; supplemented with 10% FBS) without leukemia inhibitory factor. At day 4.5, the Flk1+ MPCs were purified by AutoMACS Pro Separator (Miltenyi Biotec) with biotin-conjugated anti–mouse Flk1 antibody (clone AVAS12a1; eBioscience) and streptavidin MicroBeads (Miltenyi Biotec). The Flk1+ MPCs were then subsequently plated onto Matrigel (BD Biosciences)–coated plates at a density of 1-2 × 104 cells/cm2 and cultured in differentiation medium supplemented with VEGF-A165 (50 ng/mL, PeproTech). To purify the ECs from the differentiating Flk1+ cells, the cells were first dissociated and resuspended in HBSS/2% FBS and serially incubated with anti–mouse ICAM2 antibody (rat monoclonal, clone 3C4, BioLegend) for 10 minutes and with anti–rat IgG Microbead (Miltenyi Biotec) for 15 minutes. We then sorted the ICAM2+ cells by AutoMACS Pro Separator (Miltenyi Biotec), and subsequent FACS analysis showed that ∼ 90% of the ICAM2+ cells were CD31+/CD144+ ECs. VEGF-Trap was prepared as previously described.30 Various signaling inhibitors and activators, including SB203580, PD98059, KT5720, Go6976, KT5823, LY294002, rapamycin, Y27632, SB431542, Rho-kinase inhibitor (RKI; H-1152, isoquinolinesulfonamide), Rho-kinase inhibitor II (RKI-II; pyridyl urea), and 8-bromoadenosine 3′:5′-cyclic monophosphate (8br-cAMP) sodium salt, were purchased from Calbiochem and Sigma-Aldrich.
Immunofluorescence staining of cultured cells
Cultured cells were fixed with 2% paraformaldehyde and blocked with 5% goat (or donkey) serum in PBST (0.3% Triton X-100 in PBS) for 1 hour at room temperature. The cells were incubated overnight at 4°C with the following primary antibodies: anti–mouse CD144 (clone 11D4.1, BD Biosciences PharMingen), anti–mouse phospho-histone H3 (PHH3; rabbit polyclonal, Upstate Biotechnology), Cy3-conjugated anti–α–smooth muscle actin (α-SMA, clone 1A4, Sigma-Aldrich), and anti–mouse CD31 (clone 2H8, Chemicon). After washing in PBST 4 times, the cells were incubated for 2 hours at room temperature with the following secondary antibodies: Cy3-conjugated anti–rabbit IgG (Jackson ImmunoResearch Laboratories), Cy3-conjugated anti–hamster IgG (Jackson ImmunoResearch Laboratories), and FITC or Cy3-conjugated anti–rat (Jackson ImmunoResearch Laboratories). F-actin and nuclei were stained with TRITC-phalloidin (Invitrogen) and 4′,6-diamidino-2-phenylindole (DAPI, Invitrogen), respectively. The cells were then mounted in fluorescent mounting medium (DAKO North America). Immunofluorescent images were acquired using Zeiss LSM510 confocal fluorescence microscope (Carl Zeiss).
Flow cytometry and cell sorting
Cells were harvested with 0.25% trypsin-EDTA (Invitrogen) or dissociation buffer (Invitrogen) and resuspended in HBSS/2% FBS at 1 × 106 cells per 100 μL. The cells were incubated for 20 minutes with the following antibodies: biotin-conjugated anti–mouse Flk1 (clone AVAS12a1, eBioscience), allophycocyanin-conjugated anti–mouse CD140a (clone APA5, eBioscience), PE-conjugated anti–mouse CD31 (clone 390, eBioscience), AlexaFluor-647-conjugated anti–mouse CD144 (clone BV13, eBioscience), PE-conjugated anti–mouse Dll4 (clone HMD4-1, BioLegend), PE-conjugated anti–mouse Tie2 (clone TEK4, eBioscience), PE-conjugated anti–mouse Endoglin (clone MJ7/18, BioLegend), anti–mouse ICAM2 (clone 3C4, BioLegend), allophycocyanin-conjugated anti–mouse CD140b (clone APB5, eBioscience), PE-conjugated anti–mouse LYVE1 (clone ALY7, eBioscience), anti–mouse VEGFR3 (goat polyclonal, R&D Systems), anti–mouse EphB4 (clone VEB4-7E4, Hycult Biotech), and PE-conjugated anti–mouse Notch1 (clone HMN1-12, BioLegend). After washing in HBSS/2% FBS twice, the cells were incubated with the following secondary antibodies: PE-conjugated streptavidin (eBioscience), PE-conjugated anti–rat IgG2a (eBioscience), and FITC-conjugated anti–goat IgG (Invitrogen). Analyses and sorting were performed by FACSAria II (BD Biosciences). Dead cells were excluded by 7-aminoactinomycin D (Invitrogen). Data were analyzed using FlowJo Version 7.6.5 software (TreeStar).
EC colony assay
The Flk1+ MPCs were plated onto Matrigel-coated plate at a density of ∼ 4000 cells/cm2 and cultured in differentiation medium supplemented with VEGF-A165 (50 ng/mL) and Y27632 (10μM). At 36 hours later, the number of CD144+ EC colonies was counted. Clustering of > 5 CD144+ ECs was regarded as one EC colony.
Angiogenesis assay using a microfluidic device
To test the angiogenic property of VYI-ECs, we used a microfluidic platform that was modified from the previously described device.31,32 Briefly, a poly-dimethylsiloxane-based microfluidic platform containing 3 channels was fabricated with poly-dimethylsiloxane using a soft lithography process. As shown in supplemental Figure 7 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article), 2 empty spaces in between 3 parallel channels were filled with a mixture of type I collagen (3 mg/mL, rat tail, BD Biosciences) and fibronectin (50 μg/mL, Invitrogen), which together acted as an ECM scaffold (colored in pink). The center channel was coated with fibronectin (10 μg/mL) and was seeded with 40-50 μL of VYI-EC suspension (1 × 107 cells/mL). The device was tilted vertically for 1 hour to allow the cells to attach onto the sidewall of the scaffold by gravity. The cells were then seeded onto the other sidewall by flipping the device upside down. Cell culture media in the side channels containing VEGF-A (50 ng/mL) or VEGF-A (50 ng/mL) plus Y27632 (10μM) were exchanged with fresh media every 12 hours. Phase-contrast and immunofluorescent images were acquired to analyze the angiogenic sprouting formation of VYI-ECs.
In vivo Matrigel plug assay
A total of 1 × 106 VYI-ECs procured from iPSC (FVB strain)29 were mixed with 100 μL Matrigel supplemented with VEGF-A (500 ng/mL), and the mixture was implanted subcutaneously into the dorsal side of 6-week-old Tie2-GFP mice (FVB strain). Animal care and experimental procedures were performed with the approval of the Animal Care Committee of Korea Advanced Institute of Science and Technology. This study was conducted in accordance with the Declaration of Helsinki. After 3 weeks, the mice were killed after injection of anesthetic mixture (80 mg/kg ketamine and 12 mg/kg xylazine), and the implanted Matrigel was fixed by systemic vascular perfusion with 1% paraformaldehyde, harvested, and whole-mounted for histologic analyses.
Statistics
Values presented are mean ± SD. Significant differences between the means were determined by analysis of variance followed by the Student-Newman-Keuls test. Significance was set at P < .05 or P < .01.
Procedures
The procedures of assays for apoptosis and cell cycle, quantitative RT-PCR, Western blotting, siRNA knockdown of ROCK1 and ROCK2, Matrigel tube forming assay, and scratch wound healing assay are described in supplemental Methods.
Results
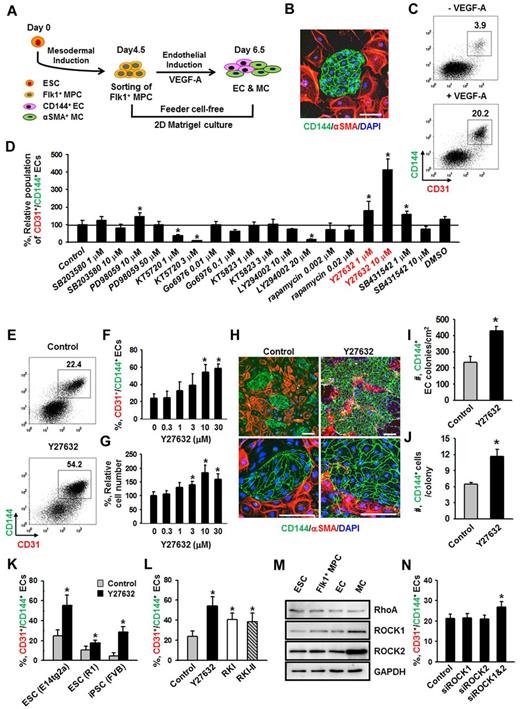
ROCK suppression promotes the differentiation and expansion of ECs from Flk1+ MPCs
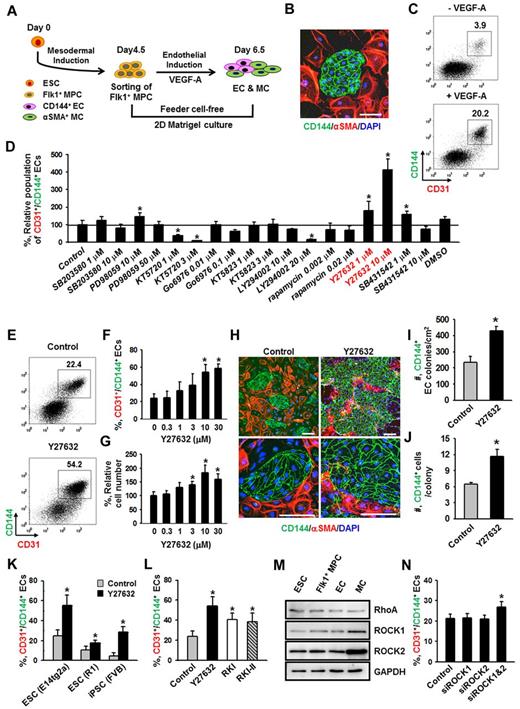
Flk1+ MPCs derived from mouse ESCs are capable of differentiating into ECs, hematopoietic cells, mural cells (MCs), and cardiomyocytes.1,4 To avoid cell contamination from using feeder cells for differentiation of Flk1+ MPCs, we have established a feeder cell-free 2D Matrigel system (Figure 1A). Flk1+ MPCs were differentiated from E14tg2a ESC cell line; and when the Flk1+ MPCs were purified at day 4.5 and cultured on Matrigel-coated plates in the differentiation medium, colonies of CD144+ ECs surrounded by α-SMA+ MCs were formed (Figure 1B). At day 6.5, the population of CD144+/CD31+ ECs grown in differentiation medium was ∼ 4%, whereas the same EC population was increased up to ∼ 20% when VEGF-A (50 ng/mL) was supplemented (Figure 1C). For the following experiments, we chose to use 50 ng/mL of VEGF-A (hereafter referred to as VEGF-A) based on its maximal effect on differentiation and expansion of ECs from Flk1+ MPCs (supplemental Figure 1A-B).
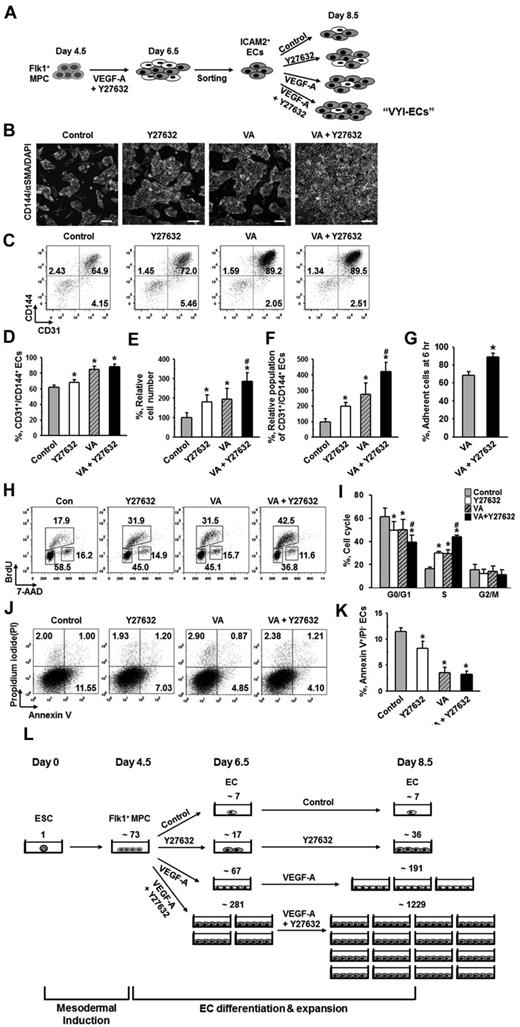
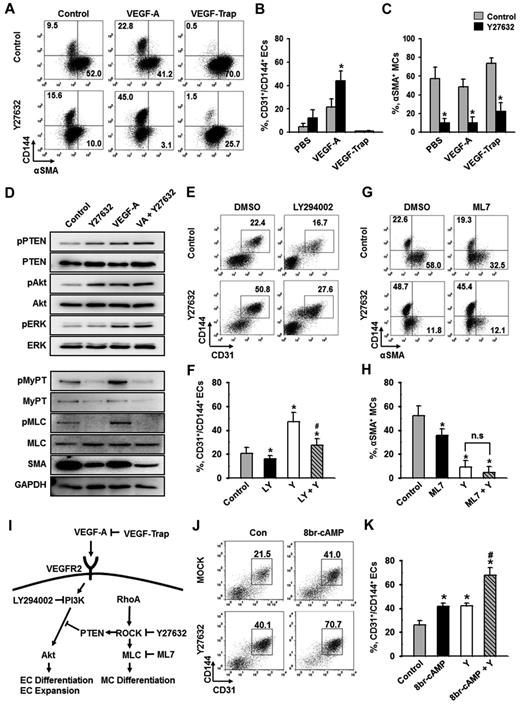
ROCK suppression promotes the differentiation and expansion of ECs from Flk1+ MPCs. (A) Protocol for differentiation of ECs. ESCs were cultured without leukemia inhibitory factor for 4.5 days for mesodermal induction. Flk1+ MPCs were then sorted by MACS and cultured with VEGF-A (50 ng/mL) for 2 days otherwise indicated in the presence of each indicated inhibitor, and their differentiation status was analyzed on day 6.5. (B) Immunofluorescence image showing CD144+ EC colonies, α-SMA+ MCs, and DAPI+ nuclei in differentiating Flk1+ MPCs. Scale bar represents 100 μm. (C) Representative FACS analysis of CD31+/CD144+ ECs incubated with or without VEGF-A(50 ng/mL). (D) Relative population of CD31+/CD144+ ECs grown with different inhibitors. Population of CD31+/CD144+ ECs grown with only VEGF-A (Control) was regarded as 100%. Each group, n = 3. *P < .01 versus Control. (E) Representative FACS analysis of CD31+/CD144+ ECs incubated with PBS (Control) or Y27632 (10μM). (F) Percentage of CD31+/CD144+ EC population incubated with various concentrations of Y27632. Each group, n = 3. *P < .01 versus 0. (G) Relative cell number of CD31+/CD144+ ECs incubated with various concentrations of Y27632. Each group, n = 3. *P < .01 versus 0. (H) Immunofluorescence images showing CD144+ EC colonies, α-SMA+ MCs, and DAPI+ nuclei in differentiating Flk1+ MPCs incubated with PBS (Control) or Y27632 (10μM). Scale bars represent 100 μm. (I-J) Comparisons of EC colony formation in a given area (cm2) and cell number in each EC colony at 36 hours after the endothelial induction. Each group, n = 4. *P < .01 versus Control. (K) Percentage of CD31+/CD144+ EC population incubated with PBS (Control) or Y27632 (10μM) in Flk1+ MPCs derived from different kinds of ESCs (E14tg2a and R1) and iPSC. Each group, n = 3. *P < .01 versus Control. (L) Percentage of CD31+/CD144+ EC population incubated with various ROCK inhibitors. Each group, n = 3. *P < .01 versus Control. (M) Immunoblotting for RhoA, ROCK1, and ROCK2 in different cell types: ESC (E14tg2a), Flk1+ MPCs, ECs, and MCs. (N) Percentage of CD31+/CD144+ EC population incubated with nonspecific control siRNA (Control), siRNA for ROCK1 (siROCK1), ROCK2 (siROCK2), or both (siROCK1 and siROCK2). Each group, n = 4. *P < .01 versus Control.
ROCK suppression promotes the differentiation and expansion of ECs from Flk1+ MPCs. (A) Protocol for differentiation of ECs. ESCs were cultured without leukemia inhibitory factor for 4.5 days for mesodermal induction. Flk1+ MPCs were then sorted by MACS and cultured with VEGF-A (50 ng/mL) for 2 days otherwise indicated in the presence of each indicated inhibitor, and their differentiation status was analyzed on day 6.5. (B) Immunofluorescence image showing CD144+ EC colonies, α-SMA+ MCs, and DAPI+ nuclei in differentiating Flk1+ MPCs. Scale bar represents 100 μm. (C) Representative FACS analysis of CD31+/CD144+ ECs incubated with or without VEGF-A(50 ng/mL). (D) Relative population of CD31+/CD144+ ECs grown with different inhibitors. Population of CD31+/CD144+ ECs grown with only VEGF-A (Control) was regarded as 100%. Each group, n = 3. *P < .01 versus Control. (E) Representative FACS analysis of CD31+/CD144+ ECs incubated with PBS (Control) or Y27632 (10μM). (F) Percentage of CD31+/CD144+ EC population incubated with various concentrations of Y27632. Each group, n = 3. *P < .01 versus 0. (G) Relative cell number of CD31+/CD144+ ECs incubated with various concentrations of Y27632. Each group, n = 3. *P < .01 versus 0. (H) Immunofluorescence images showing CD144+ EC colonies, α-SMA+ MCs, and DAPI+ nuclei in differentiating Flk1+ MPCs incubated with PBS (Control) or Y27632 (10μM). Scale bars represent 100 μm. (I-J) Comparisons of EC colony formation in a given area (cm2) and cell number in each EC colony at 36 hours after the endothelial induction. Each group, n = 4. *P < .01 versus Control. (K) Percentage of CD31+/CD144+ EC population incubated with PBS (Control) or Y27632 (10μM) in Flk1+ MPCs derived from different kinds of ESCs (E14tg2a and R1) and iPSC. Each group, n = 3. *P < .01 versus Control. (L) Percentage of CD31+/CD144+ EC population incubated with various ROCK inhibitors. Each group, n = 3. *P < .01 versus Control. (M) Immunoblotting for RhoA, ROCK1, and ROCK2 in different cell types: ESC (E14tg2a), Flk1+ MPCs, ECs, and MCs. (N) Percentage of CD31+/CD144+ EC population incubated with nonspecific control siRNA (Control), siRNA for ROCK1 (siROCK1), ROCK2 (siROCK2), or both (siROCK1 and siROCK2). Each group, n = 4. *P < .01 versus Control.
To dissect the signaling pathways involved in VEGF-A–induced differentiation and expansion of ECs, various kinds of signaling inhibitors [PI3-kinase inhibitor (LY294002), MEK/ERK inhibitor (PD98059), p38 inhibitor (SB203580), PKA inhibitor (KT5720), PKC inhibitor (Go6976), PKG inhibitor (KT5823), mTOR inhibitor (rapamycin), TGF-β inhibitor (SB431542), and ROCK inhibitor (Y27632)] were treated with VEGF-A, and the population of CD31+/CD144+ ECs was analyzed at day 6.5 (Figure 1D). Among them, PKA inhibitor and PI3-kinase inhibitor strongly reduced the CD31+/CD144+ EC population, indicating that the VEGF-A–induced differentiation of ECs mainly results from activation of PKA and PI3-kinase, which is in agreement with the previous report.8 To our surprise, we noticed that ROCK inhibitor Y27632 (1 and 10μM) dramatically increased the CD31+/CD144+ EC population (1.8- and 4.1-fold, respectively, compared with Control). Y27632 treatment simultaneously increased not only the percentage of EC population, but also the total cell number in a dose-dependent manner (Figure 1E-G). Accordingly, we were able to amplify the total number of CD31+/CD144+ ECs from Flk1+ MPCs by ∼ 4.2-fold through treatment of 10μM of Y27632 plus VEGF-A compared with treatment of only VEGF-A (Control). We chose 10μM of Y27632 (hereafter referred to as Y27632 otherwise indicated) for the following experiments based on its maximum effect on the promotion of EC population. Immunofluorescent staining analysis on day 6.5 showed that Y27632 increased both the size and number of CD144+ EC colonies, whereas it decreased the number of surrounding α-SMA+ MCs (Figure 1H; see also supplemental Videos 1 and 2). In addition, EC colony assay revealed that Y27632 increased the number of EC colonies as well as the cell number in each colony (Figure 1I-J; supplemental Figure 2A-B). These data indicate that Y27632 promotes not only the differentiation of ECs from Flk1+ MPCs but also the expansion of the induced ECs. On the other hand, Y27632 slightly reduced the induction of Flk1+ MPCs from ESCs (supplemental Figure 2C-F).
Aside from the E14tg2a cell line used for endothelial differentiation, Y27632 also promoted differentiation and expansion of ECs from R1 ESC cell line and iPSC by 1.6- and 5.9-fold compared with each control, respectively, suggesting that this procedure can be applied to Flk1+ MPCs derived from any source of mouse ESCs (Figure 1K). To confirm that the effect of Y27632 is truly via suppression of ROCK signaling, we applied 2 different ROCK inhibitors (RKI and RKI-II); both RKI and RKI-II similarly increased the EC population, indicating that promotion of the EC population is indeed a result of suppression of ROCK signaling (Figure 1L). Indeed, both ROCK1 and ROCK2 and their main upstream signaling molecule RhoA were expressed in ESCs and Flk1+ MPCs as well as in ECs and MCs (Figure 1M). To find the ROCK subtype responsible for the increase in EC population, we depleted ROCK1, ROCK2, or both by treatment of their respective siRNAs into the Flk1+ MPCs. Although treatment of either siROCK1 or siROCK2 alone did not promote EC population, the combination of siROCK1 and siROCK2 significantly promoted the EC population (Figure 1N). Our interpretation of this finding is that blockade of one subtype of ROCK up-regulates the other type of ROCK and negates the siRNA-mediated blocking effect, so that blockade of both types of ROCK is required for the promotion of the EC population.
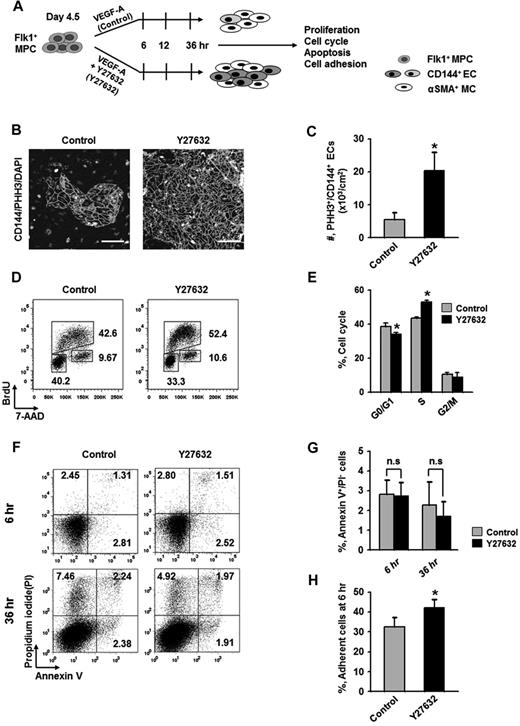
Expansion of differentiated ECs by ROCK suppression is mainly the result of the promotion of EC proliferation
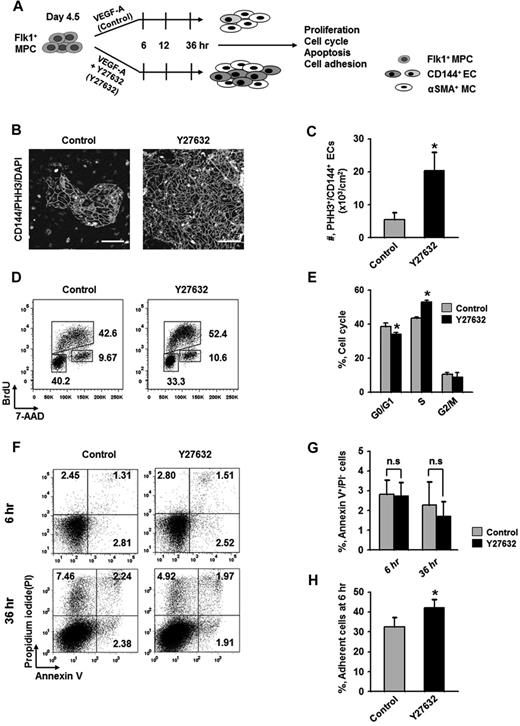
To elucidate how ROCK suppression promotes the differentiation and expansion of ECs, we examined the effect of Y27632 on the proliferation, dissociation-induced apoptosis (also regarded as “anoikis”) at 6 hours, apoptosis at 36 hours, and adhesion during differentiation (Figure 2A). BrdU incorporation assay revealed that during the differentiation process of ECs, Y27632 increased BrdU+/CD144+ EC population by 1.7-fold and 2.7-fold at 12 and 36 hours, respectively (supplemental Figure 3). PHH3 immunostaining also revealed that Y27632 increased the population of PHH3+/CD144+ ECs by 3.7-fold compared with control at 36 hours (Figure 2B-C). Accordingly, BrdU/7-aminoactinomycin D analysis revealed that, compared with control, Y27632 increased the population of CD144+ ECs in S phase by 1.23-fold, whereas it reduced those in G0/G1 phase by 12% at 36 hours (Figure 2D-E). However, Y27632 did not significantly change the population of annexin V+/propidium iodide (PI)− apoptotic cells at 6 and 36 hours (Figure 2F-G). Notably, Y27632 promoted the adhesion of Flk1+ cells onto the plates by 1.3-fold compared with control at 6 hours (Figure 2H). We observed that, at 48 hours after removal of the nonadherent cells at 6 hours, Y27632 also simultaneously increased the percentage of EC population by 1.4-fold compared with control (supplemental Figure 4). Thus, the expansion of differentiated EC by ROCK suppression is mainly the result of the combinative promotion of proliferation and adhesion of CD144+ ECs, rather than the suppression of anoikis and apoptosis of the same.
Expansion of differentiated ECs by ROCK suppression is mainly the result of the promotions of EC proliferation and Flk1+ MPC adhesion. (A) Diagram of time points (6, 12, and 36 hours) for assays of proliferation, cell cycle, apoptosis, and adhesion in differentiating Flk1+ MPCs incubated with VEGF-A (50 ng/mL; Control) or VEGF-A (50 ng/mL) plus Y27632 (10μM; Y72632). (B) Immunofluorescence images showing PHH3+/CD144+ proliferative EC colonies and DAPI+ nuclei at 36 hours. Scale bars represent 100 μm. (C) Number of PHH3+/CD144+ ECs in cultured area (cm2) at 36 hours. Each group, n = 5. *P < .05 versus Control. (D) Representative FACS analysis showing cell cycle in CD144+ ECs from differentiating Flk1+ MPCs at 36 hours. (E) Percentage of CD144+ ECs under each phase of cell cycle at 36 hours. Each group, n = 3. *P < .01 versus Control. (F) Representative FACS analysis showing annexin V+/ PI− apoptotic cells. (G) Percentage of annexin V+/PI− apoptotic cells at 6 and 36 hours. Each group, n = 4. n.s represents statistically nonsignificant. (H) Percentage of adherent cells onto plate at 6 hours. Each group, n = 5. *P < .05 versus Control.
Expansion of differentiated ECs by ROCK suppression is mainly the result of the promotions of EC proliferation and Flk1+ MPC adhesion. (A) Diagram of time points (6, 12, and 36 hours) for assays of proliferation, cell cycle, apoptosis, and adhesion in differentiating Flk1+ MPCs incubated with VEGF-A (50 ng/mL; Control) or VEGF-A (50 ng/mL) plus Y27632 (10μM; Y72632). (B) Immunofluorescence images showing PHH3+/CD144+ proliferative EC colonies and DAPI+ nuclei at 36 hours. Scale bars represent 100 μm. (C) Number of PHH3+/CD144+ ECs in cultured area (cm2) at 36 hours. Each group, n = 5. *P < .05 versus Control. (D) Representative FACS analysis showing cell cycle in CD144+ ECs from differentiating Flk1+ MPCs at 36 hours. (E) Percentage of CD144+ ECs under each phase of cell cycle at 36 hours. Each group, n = 3. *P < .01 versus Control. (F) Representative FACS analysis showing annexin V+/ PI− apoptotic cells. (G) Percentage of annexin V+/PI− apoptotic cells at 6 and 36 hours. Each group, n = 4. n.s represents statistically nonsignificant. (H) Percentage of adherent cells onto plate at 6 hours. Each group, n = 5. *P < .05 versus Control.
ROCK suppression promotes the differentiation of ECs through PTEN-Akt signaling and inhibits the differentiation of MCs through MyPT-MLC signaling
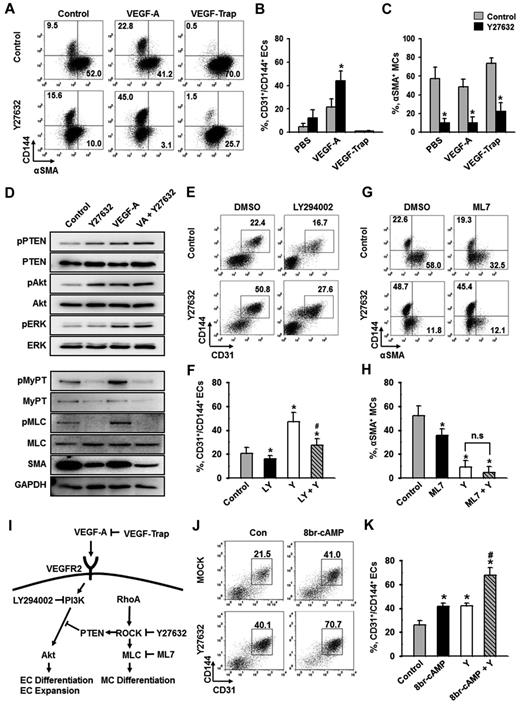
To elucidate the downstream signaling pathway responsible for ROCK suppression-induced differentiation and expansion of ECs, we examined the extent of phosphorylation of several key signaling molecules. VEGF-A phosphorylated VEGFR2 in Flk1+ MPCs and activated its major downstream signaling pathways, ERK and Akt (supplemental Figure 1C), indicating that VEGF-A/VEGFR2 signaling is active and dynamic in Flk1+ MPCs during the EC differentiation process. To investigate whether the ROCK suppression-induced EC expansion is VEGF-A dependent or not, we blocked the action of VEGF-A through pretreatment of VEGF-Trap during the differentiation (Figure 3A-B). VEGF-Trap totally abolished the ROCK suppression-induced expansion of ECs, indicating that the process is indeed VEGF-A dependent. Notably, ROCK suppression profoundly reduced α-SMA+ MC populations whether or not VEGF-A was supplemented or blocked (Figure 3A,C). In other words, ROCK suppression alone significantly inhibits MC differentiation, and the combination of ROCK suppression and VEGF-A stimulation is able to synergistically promote the differentiation and expansion of ECs (Figure 3I). These data suggest that ROCK downstream signaling pathway responsible for EC differentiation could be separate from that for MC differentiation.
PI3-kinase/Akt signaling pathway, but not cAMP, is involved in ROCK suppression-induced expansion of ECs. Flk1+ MPCs were incubated with the indicated agents for 2 days, and analyses were performed on day 6.5. (A) Representative FACS analysis showing CD144+ ECs or α-SMA+ MCs incubated with PBS (Control) or Y27632 (10μM) in the absence or presence of VEGF-A (50 ng/mL) and VEGF-Trap (5 μg/mL). (B-C) Percentage of CD31+/CD144+ EC population and α-SMA+ MC population. (B) Each group, n = 3. *P < .01 versus Control. (C) Each group, n = 4. *P < .01 versus each Control. (D) Immunoblotting for pPTEN, PTEN, pAkt, Akt, pERK, ERK, pMyPT, MyPT, pMLC, MLC, α-SMA, and GAPDH in Flk1+ MPCs treated with PBS (Control), Y27632 (10μM), VEGF-A (50 ng/mL; VA), or VA plus Y27632. The Flk1+ MPCs were cultured for 12 hours, starved for 6 hours with or without Y27632, and then treated with VEGF-A (50 ng/mL) for 10 minutes. Results from 4 independent trials were similar to each other. (E) Representative FACS analysis of CD31+/CD144+ ECs incubated with 0.1% DMSO or LY294002 (20μM; LY) in the presence of PBS (Control) or Y27632 (10μM; Y). (F) Percentage of CD31+/CD144+ EC population. Each group, n = 3. *P < .01 versus DMSO; #P < .01 versus Y27632. (G) Representative FACS analysis of α-SMA+ MCs or CD144+ ECs incubated with 0. 1% DMSO or ML7 (5μM) in the presence of PBS (Control) or Y27632 (10μM). (H) Percentage of α-SMA+ MC population. Each group, n = 3. *P < .01 versus DMSO. n.s represents statistically nonsignificant. (I) Schematic diagram showing the negative involvement of ROCK in VEGF-A/VEGFR2/PI3-kinase/AKT signaling pathway via PTEN in differentiation of ECs and the positive involvement of ROCK in differentiation of MCs via MLC in the Flk1+ MPCs. Specific inhibitors for each signaling molecules are indicated. (J) Representative FACS analysis of CD31+/CD144+ ECs incubated with PBS (Control) or 8br-cAMP (0.5mM) in the presence of PBS (Control) or Y27632 (10μM; Y). (K) Percentage of CD31+/CD144+ EC population. Each group, n = 4. *P < .01 versus Control. #P < .01 versus 8br-cAMP or Y27632.
PI3-kinase/Akt signaling pathway, but not cAMP, is involved in ROCK suppression-induced expansion of ECs. Flk1+ MPCs were incubated with the indicated agents for 2 days, and analyses were performed on day 6.5. (A) Representative FACS analysis showing CD144+ ECs or α-SMA+ MCs incubated with PBS (Control) or Y27632 (10μM) in the absence or presence of VEGF-A (50 ng/mL) and VEGF-Trap (5 μg/mL). (B-C) Percentage of CD31+/CD144+ EC population and α-SMA+ MC population. (B) Each group, n = 3. *P < .01 versus Control. (C) Each group, n = 4. *P < .01 versus each Control. (D) Immunoblotting for pPTEN, PTEN, pAkt, Akt, pERK, ERK, pMyPT, MyPT, pMLC, MLC, α-SMA, and GAPDH in Flk1+ MPCs treated with PBS (Control), Y27632 (10μM), VEGF-A (50 ng/mL; VA), or VA plus Y27632. The Flk1+ MPCs were cultured for 12 hours, starved for 6 hours with or without Y27632, and then treated with VEGF-A (50 ng/mL) for 10 minutes. Results from 4 independent trials were similar to each other. (E) Representative FACS analysis of CD31+/CD144+ ECs incubated with 0.1% DMSO or LY294002 (20μM; LY) in the presence of PBS (Control) or Y27632 (10μM; Y). (F) Percentage of CD31+/CD144+ EC population. Each group, n = 3. *P < .01 versus DMSO; #P < .01 versus Y27632. (G) Representative FACS analysis of α-SMA+ MCs or CD144+ ECs incubated with 0. 1% DMSO or ML7 (5μM) in the presence of PBS (Control) or Y27632 (10μM). (H) Percentage of α-SMA+ MC population. Each group, n = 3. *P < .01 versus DMSO. n.s represents statistically nonsignificant. (I) Schematic diagram showing the negative involvement of ROCK in VEGF-A/VEGFR2/PI3-kinase/AKT signaling pathway via PTEN in differentiation of ECs and the positive involvement of ROCK in differentiation of MCs via MLC in the Flk1+ MPCs. Specific inhibitors for each signaling molecules are indicated. (J) Representative FACS analysis of CD31+/CD144+ ECs incubated with PBS (Control) or 8br-cAMP (0.5mM) in the presence of PBS (Control) or Y27632 (10μM; Y). (K) Percentage of CD31+/CD144+ EC population. Each group, n = 4. *P < .01 versus Control. #P < .01 versus 8br-cAMP or Y27632.
RhoA-ROCK signaling is known to stimulate the phosphatase activity of PTEN, leading to suppression of Akt activation in leukocytes and human embryonic kidney cells.33 In agreement with this phenomenon, ROCK suppression via Y27632-phosphorylated PTEN (Ser380, Thr382, and Thr383) and Akt (Ser473) in the Flk1+ MPCs, but not ERK (Figure 3D; supplemental Figure 5A). In comparison, VEGF-A alone or VEGF-A plus Y27632 phosphorylated all PTEN, Akt, and ERK (Figure 3D; supplemental Figure 5A). Accordingly, PI3-kinase inhibitor LY294002 almost completely suppressed the ROCK suppression-induced differentiation and expansion of ECs under VEGF-A stimulus (Figure 3E-F). This demonstrates that ROCK suppression-induced PTEN inhibition promotes VEGF-A–induced PI3-kinase-Akt signaling, leading to enhanced proliferation, differentiation, and expansion of ECs (Figure 3I).
MyPT and myosin light chain (MLC) are crucial elements for myogenic differentiation, both of which are regulated by ROCK.23-25 Indeed, ROCK suppression via Y27632 almost completely reduced the phosphorylation of MyPT and MLC and, in parallel, reduced the protein levels of α-SMA regardless of VEGF-A stimulation in Flk1+ MPCs (Figure 3D; supplemental Figure 5A). Indeed, Y27632 reduced α-SMA+ MC population by ∼ 83% and MLC kinase inhibitor ML7 (5μM) reduced α-SMA+ MC population by ∼ 31%, and combination of Y27632 and ML7 further reduced α-SMA+ MC population by ∼ 91% (Figure 3G-H). This collectively shows that ROCK suppression reduces the differentiation of MCs from Flk1+ MPCs through inhibition of MyPT-MLC signaling pathway (Figure 3I).
We also evaluated the effect of 8br-cAMP, cell membrane permeable cAMP, on the differentiation and expansion of ECs based on the report that intracelluar cAMP enhances VEGF-A–induced EC differentiation.7 Supplemental 8br-cAMP (0.5mM) increased the CD31+/CD144+ EC population to a similar degree as Y27632, and the combination of these 2 compounds produced an additive effect in EC population (Figure 3J-K), indicating that cAMP and Y27632 promote the differentiation and expansion of ECs through distinct mechanisms.
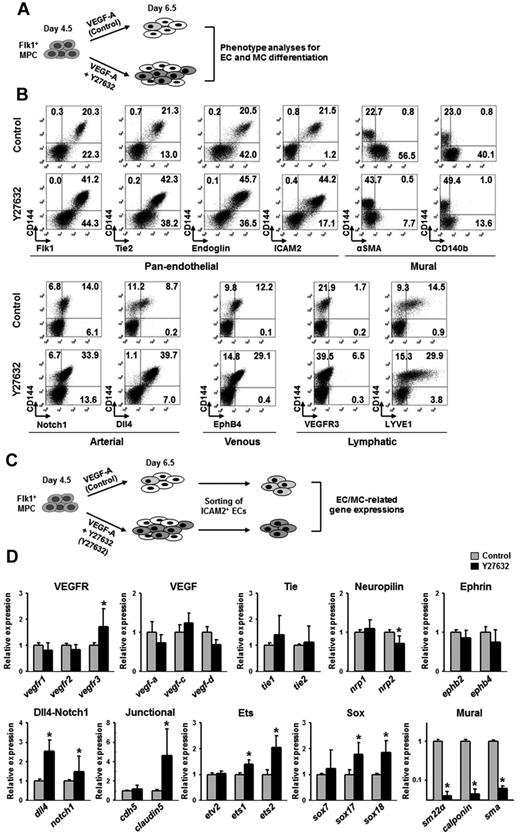
ROCK suppression-induced ECs express typical EC markers and EC-related genes
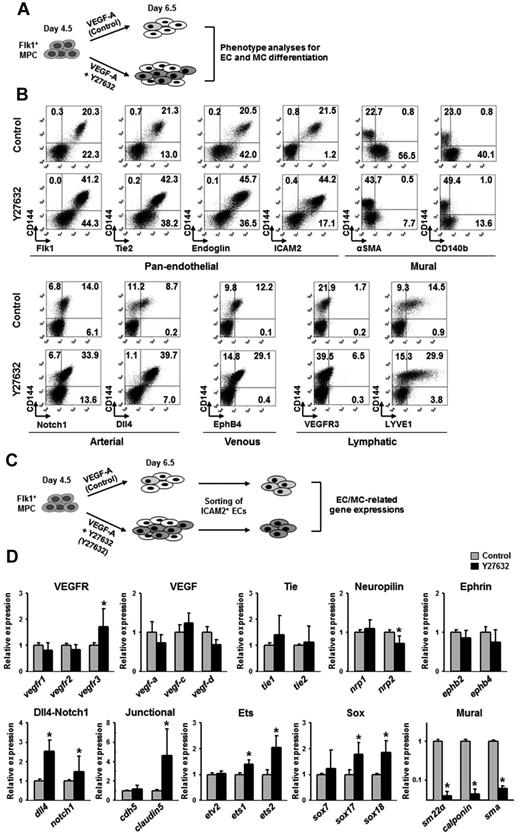
To characterize the ECs differentiated and expanded through ROCK suppression, we performed phenotypic analyses of various EC and MC markers at day 6.5 using FACS (Figure 4A). Compared with control, Y27632 increased the population of CD144+ ECs expressing pan-endothelial cell surface makers (Flk1, Tie2, endoglin, and ICAM2) by ∼ 2.0-fold, whereas it markedly reduced the populations of cells expressing α-SMA and CD140b (Figure 4B; supplemental Figure 5B). Further analyses revealed that Y27632 increased the population of CD144+ ECs that express Notch1 (arterial, 2.4-fold), Dll4 (arterial, 4.6-fold), EphB4 (venous, 2.4-fold), VEGFR3 (lymphatic, 3.8-fold), and LYVE-1 (lymphatic, 2.1-fold; Figure 4B; supplemental Figure 5C). At day 6.5, most CD144+ cells coexpressed intercellular adhesion molecule-2 (ICAM2), a surface marker for ECs that is able to remain in its intact form after dissociation of cultured cells by trypsin-EDTA treatment. Taking the advantage of remaining surface expression of ICAM2,34 we were able to isolate ICAM2+ ECs by MACS at a purity of ∼ 90% (Figure 4C). We then further analyzed the mRNA expression of EC- and MC-related genes in the purified ICAM2+ cells (Figure 4C). The ICAM2+ ECs induced by VEGF-A expressed typical EC-specific genes, VEGF ligands, VEGF receptors, Tie receptors, neuropilins, ephrinB2-EphB4, Dll4-Notch1, EC-specific junctional proteins, and Ets and Sox family transcriptional factors, as well as MC-specific genes, including SM22a, calponin, and SMA. Compared with the ICAM2+ ECs induced by VEGF-A, the ICAM2+ ECs induced by VEGF-A plus Y27632 expressed more vegfr3 (1.7-fold), dll4 (2.5-fold), notch1 (1.5-fold), claudin-5 (4.6-fold), ets1 (1.4-fold), ets2 (2.1-fold), sox17 (1.7-fold), and sox18 (1.8-fold), whereas they showed drastic reduced expression level of all SMC-specific genes: sm22a (25-fold), calponin (25-fold), and sma (17-fold; Figure 4D). Our interpretation of this finding is that the ICAM2+ ECs induced by VEGF-A plus Y27632 are further differentiated toward arterial ECs compared with those induced by VEGF-A only.
ROCK suppression-induced ECs express EC-specific genes. (A) Diagram of cell preparations for phenotype analyses of EC and MC differentiation. Flk1+ MPCs were incubated with only VEGF-A (50 ng/mL; Control) and VEGF-A (50 ng/mL) plus Y27632 (10μM; Y27632) for 2 days. (B) Representative FACS analysis for phenotypic markers in CD144+ cells; pan-endothelial, mural, and arterial, venous, and lymphatic ECs. (C) Diagram of cell preparations for analyses of gene expression related to differentiation of ECs and MCs. The cells in diagram panel A were sorted with anti-ICAM2 antibody, and gene expressions in each population of ICAM2+ ECs were analyzed by quantitative RT-PCR. (D) Comparisons of EC and MC differentiation-related gene expressions. Each group, n = 3. *P < .01 versus each Control.
ROCK suppression-induced ECs express EC-specific genes. (A) Diagram of cell preparations for phenotype analyses of EC and MC differentiation. Flk1+ MPCs were incubated with only VEGF-A (50 ng/mL; Control) and VEGF-A (50 ng/mL) plus Y27632 (10μM; Y27632) for 2 days. (B) Representative FACS analysis for phenotypic markers in CD144+ cells; pan-endothelial, mural, and arterial, venous, and lymphatic ECs. (C) Diagram of cell preparations for analyses of gene expression related to differentiation of ECs and MCs. The cells in diagram panel A were sorted with anti-ICAM2 antibody, and gene expressions in each population of ICAM2+ ECs were analyzed by quantitative RT-PCR. (D) Comparisons of EC and MC differentiation-related gene expressions. Each group, n = 3. *P < .01 versus each Control.
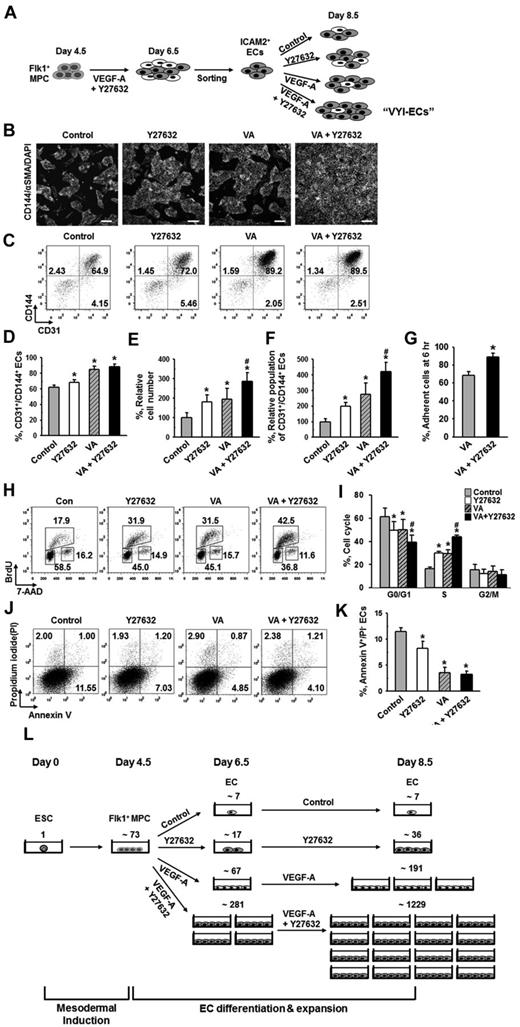
ROCK suppression continuously promotes the expansion of ECs
To maximize the amount of ECs obtained from ESCs, we expanded the ICAM2+ ECs for 2 days using the same culture system with VEGF-A and Y27632 (Figure 5A). When the ECs were grown for 2 days without either VEGF-A or Y27632, the population of CD31+/CD144+ ECs underwent a decrease of ∼ 30% from the initial population of ∼ 90% (Figure 5B-D). This decrease in EC population was dramatically ameliorated by treatment of VEGF-A alone, in which case the CD31+/CD144+ population was only decreased by ∼ 5% instead of ∼ 30%. In contrast, treatment of Y27632 did not significantly affect the decrease of EC population: the EC population was decreased by ∼ 22% when Y27632 was treated alone, respectively (Figure 5B-D). On the other hand, Y27632 alone or VEGF-A alone similarly increased the total cell number by ∼ 1.8-fold and ∼ 1.9-fold, respectively, and the combination of Y27632 and VEGF-A further increased the total cell number by ∼ 2.9-fold (Figure 5E). This way, we were further able to obtain ∼ 4.2-fold expansion of CD31+/CD144+ ECs from the ICAM2+ ECs (Figure 5B-F). In the presence of VEGF-A, Y27632 promoted the adhesion of ICAM2+ ECs onto the plate by 1.3-fold at 6 hours after plating (Figure 5G). In these purified ECs, Y27632 and VEGF-A each promoted the proliferation of ECs to a similar level, and the combination of Y27632 and VEGF-A promoted EC proliferation to a greater degree (supplemental Figure 6). We also performed FACS analysis to examine the cell cycle of CD144+ ECs in ICAM2+ ECs at 12 hours after plating and found that, compared with control, Y27632 and VEGF-A each increased the percentage of the cells in S phase to a similar degree (∼ 1.8-fold) and that the combination of the 2 compounds further increased the percentage of proliferating cells by 2.7-fold (Figure 5H-I). Furthermore, we noted that Y27632 mildly inhibited the apoptosis of ECs, whereas VEGF-A strongly inhibited the same (Figure 5J-K). Based on these findings, we conclude that further expansion of high-purity ECs (> 90%) could be achieved from ESCs by combination of simultaneous ROCK suppression and VEGF-stimulation: these high-purity ECs are hereafter referred to as “VEGF-A plus Y27 632-induced and anti-ICAM2 antibody-sorted ECs (VYI-ECs)” (Figure 5A). We estimated how many VYI-ECs can be obtained at day 8.5 from 1 ESC using our 2D Matrigel system: from ∼ 73 Flk1+ MPCs differentiated from 1 ESC, only ∼ 36 ECs and ∼ 191 ECs were generated by treatment of either Y27632 or VEGF-A alone, respectively; in contrast, ∼ 1229 ECs were generated by combination of Y27632 and VEGF-A (Figure 5L).
ROCK suppression continuously promotes the expansion of ECs. (A) ICAM2+ ECs were further incubated with PBS (Control), Y27632 (10μM), VEGF-A (50 ng/mL; VA), or Y27632 (10μM) plus VEGF-A (50 ng/mL) for 2 days, and analyses were performed at day 8.5. (B) Immunofluorescence images showing CD144+ EC colonies, α-SMA+ MCs, and DAPI+ nuclei. Scale bars represent 100 μm. (C) Representative FACS analysis of CD31+/CD144+ ECs. (D) Percentage of CD31+/CD144+ EC population. Each group, n = 3. *P < .01 versus Control. (E) Relative cell number of ICAM2+ ECs. Each group, n = 3. *P < .01 versus Control. #P < .01 versus VEGF-A. (F) Relative population of CD31+/CD144+ ECs. Population of CD31+/CD144+ ECs grown without VEGF-A and Y27632 was regarded as 100%. Each group, n = 3. *P < .01 versus Control. #P < .01 versus VEGF-A. (G) Percentage of adherent ICAM2+ ECs onto plate at 6 hours. Each group, n = 3. *P < .05 versus VEGF-A. (H) Representative FACS analysis showing cell cycle in ICAM2+ ECs at 12 hours. (I) Percentage of ICAM2+ ECs under each phase of cell cycle at 12 hours. Each group, n = 3. *P < .01 versus Control. #P < .01 versus VEGF-A. (J) Representative FACS analysis of annexin V+/PI− apoptotic ECs. (K) Percentage of annexin V+/PI− ECs. Each group, n = 3. *P < .01 versus Control. (L) Estimation and comparison of number of ECs generated from one ESC grown with indicated agents in the 2D Matrigel system at day 6.5 and 8.5. Estimation was calculated from 5 independent incubations.
ROCK suppression continuously promotes the expansion of ECs. (A) ICAM2+ ECs were further incubated with PBS (Control), Y27632 (10μM), VEGF-A (50 ng/mL; VA), or Y27632 (10μM) plus VEGF-A (50 ng/mL) for 2 days, and analyses were performed at day 8.5. (B) Immunofluorescence images showing CD144+ EC colonies, α-SMA+ MCs, and DAPI+ nuclei. Scale bars represent 100 μm. (C) Representative FACS analysis of CD31+/CD144+ ECs. (D) Percentage of CD31+/CD144+ EC population. Each group, n = 3. *P < .01 versus Control. (E) Relative cell number of ICAM2+ ECs. Each group, n = 3. *P < .01 versus Control. #P < .01 versus VEGF-A. (F) Relative population of CD31+/CD144+ ECs. Population of CD31+/CD144+ ECs grown without VEGF-A and Y27632 was regarded as 100%. Each group, n = 3. *P < .01 versus Control. #P < .01 versus VEGF-A. (G) Percentage of adherent ICAM2+ ECs onto plate at 6 hours. Each group, n = 3. *P < .05 versus VEGF-A. (H) Representative FACS analysis showing cell cycle in ICAM2+ ECs at 12 hours. (I) Percentage of ICAM2+ ECs under each phase of cell cycle at 12 hours. Each group, n = 3. *P < .01 versus Control. #P < .01 versus VEGF-A. (J) Representative FACS analysis of annexin V+/PI− apoptotic ECs. (K) Percentage of annexin V+/PI− ECs. Each group, n = 3. *P < .01 versus Control. (L) Estimation and comparison of number of ECs generated from one ESC grown with indicated agents in the 2D Matrigel system at day 6.5 and 8.5. Estimation was calculated from 5 independent incubations.
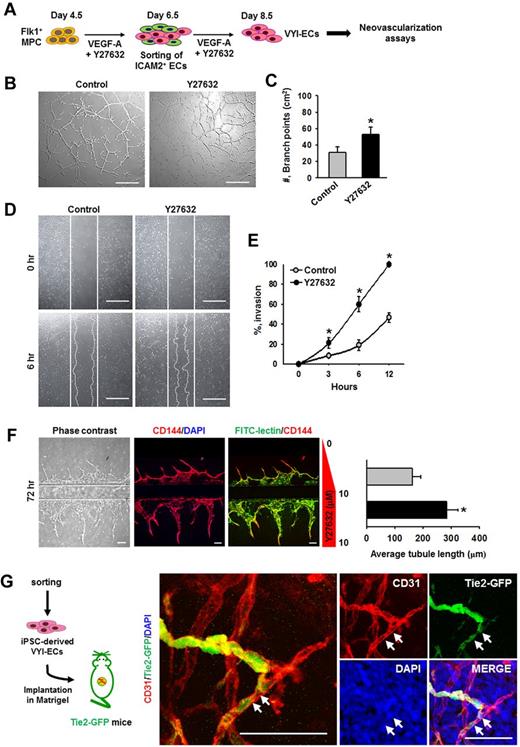
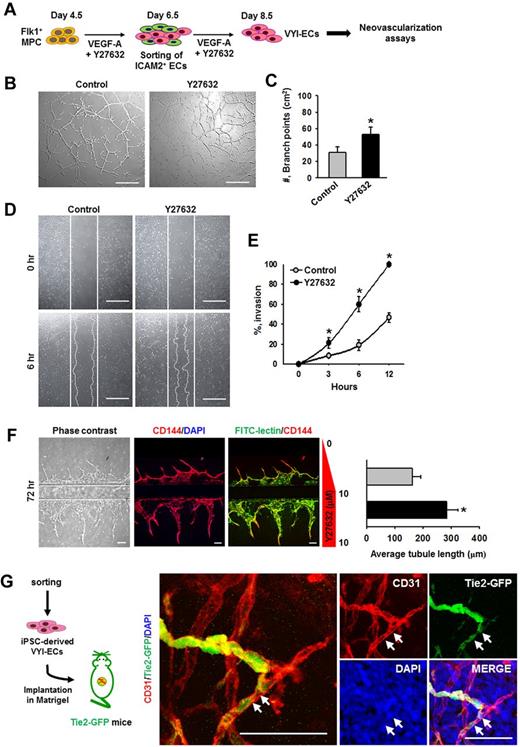
VYI-ECs have neovasculogenic capabilities in vitro and in vivo
To determine the in vitro neovasculogenic properties of VYI-ECs, various assays, including tube formation, scratch wound healing, and angiogenic ability using a microfluidic system, were performed (Figure 6A). Treatment of VEGF-A alone (Control) induced the formation of vascular network (∼ 31.3 ± 6.4/cm2, measured by counting the number of branch points) at 12 hours after VYI-ECs have been plated (Figure 6B); VEGF-A also induced a gradual invasion of ECs into the wound region (∼ 46.7% ± 4.9% at 12 hours; Figure 6D). The addition of Y27632 increased the formation of vascular networks (1.7-fold) as well as cell invasion into the wound region (2.1-fold; Figure 6B-E). We also designed a “microfluidic angiogenesis assay system” that allowed us to assess the angiogenic property of VYI-ECs (supplemental Figure 7), and we observed that cotreatment of Y27632 increased the length of sprouting CD144+ VYI-ECs by 1.7-fold at 72 hours (Figure 6F).29 In vivo neovasculogenic potential of VYI-ECs derived from iPSCs (FVB origin) was tested using a Matrigel plug implantation into Tie2-GFP+ mice (FVB origin),35 of which the blood vessels and a subset of hematopoietic cells of the recipient mouse are labeled with GFP to distinguish the donor-derived vessels from recipient-derived vessels. Immunofluorescent staining showed both recipient-derived GFP+/CD31+ vessels and the implanted VYI-EC–derived GFP−/CD31+ vessels in the Matrigel; noticeably, some of the VYI-EC–derived vessels were connected to the adjacent recipient blood vessels (Figure 6G), suggesting that VYI-ECs were successfully incorporated with the recipient circulatory network. Based on these observations, we conclude that VYI-ECs have neovasculogenic capabilities in vivo as well as in vitro.
VYI-ECs have neovasculogenic capabilities in vitro and in vivo. (A) Diagram of VYI-EC preparation for analyses of neovasculogenic activities. VYI-ECs were applied for tube-forming assay, scratch wound healing assay, microfluidic angiogenesis assay using VEGF-A (50 ng/mL; Control) or VEGF-A (50 ng/mL) plus Y27632 (10μM; Y72632) as supplemental materials, and in vivo Matrigel plug implantation assay. (B) Representative phase-contrast images showing network and branch formations of VYI-ECs at 12 hours. Scale bars represent 100 μm. (C) Number of branch points in a given area (cm2). Branch point was defined as the contact point of 3 or more endothelial tubes. Each group, n = 3. *P < .05 versus Control. (D) Representative phase-contrast images showing invasion of VYI-ECs (white dotted lines) into wound regions at 6 hours. White solid lines indicate the boundaries of wounding. Scale bars represent 100 μm. (E) Percentage of invasions. Area of wound at 0 hours is regarded as 100%. Each group, n = 3. *P < .05 versus Control. (F left): Representative phase-contrast and immunofluorescence images showing the sprouting of CD144+/FITC-lectin+ ECs into ECM scaffold at 48 hours after cell seeding. Upper ECM scaffold provides a negative gradient of Y27632 (0-10μM; Control), whereas lower ECM scaffold provides a constant concentration of Y27632 (10μM; Y27632). Nuclei were stained with DAPI. Scale bars represent 100 μm. (Right): Average length of sprouting ECs. *P < .05 versus Control. (G) Diagram of in vivo Matrigel plug assay. VYI-ECs derived from iPSCs were mixed with Matrigel supplemented with VEGF-A (500 ng/mL) and implanted into the dorsal flank of Tie2-GFP mouse. Implanted Matrigel was immunostained for CD31+ blood vessels and stained for DAPI+ nuclei. Donor-derived CD31+/GFP− blood vessels are formed in the gel, whereas invasion of recipient-derived CD31+/GFP+ blood vessels into the gel is detected. White arrows indicate the implanted VYI-ECs that have been integrated into recipient vessel. Scale bars represent 50 μm.
VYI-ECs have neovasculogenic capabilities in vitro and in vivo. (A) Diagram of VYI-EC preparation for analyses of neovasculogenic activities. VYI-ECs were applied for tube-forming assay, scratch wound healing assay, microfluidic angiogenesis assay using VEGF-A (50 ng/mL; Control) or VEGF-A (50 ng/mL) plus Y27632 (10μM; Y72632) as supplemental materials, and in vivo Matrigel plug implantation assay. (B) Representative phase-contrast images showing network and branch formations of VYI-ECs at 12 hours. Scale bars represent 100 μm. (C) Number of branch points in a given area (cm2). Branch point was defined as the contact point of 3 or more endothelial tubes. Each group, n = 3. *P < .05 versus Control. (D) Representative phase-contrast images showing invasion of VYI-ECs (white dotted lines) into wound regions at 6 hours. White solid lines indicate the boundaries of wounding. Scale bars represent 100 μm. (E) Percentage of invasions. Area of wound at 0 hours is regarded as 100%. Each group, n = 3. *P < .05 versus Control. (F left): Representative phase-contrast and immunofluorescence images showing the sprouting of CD144+/FITC-lectin+ ECs into ECM scaffold at 48 hours after cell seeding. Upper ECM scaffold provides a negative gradient of Y27632 (0-10μM; Control), whereas lower ECM scaffold provides a constant concentration of Y27632 (10μM; Y27632). Nuclei were stained with DAPI. Scale bars represent 100 μm. (Right): Average length of sprouting ECs. *P < .05 versus Control. (G) Diagram of in vivo Matrigel plug assay. VYI-ECs derived from iPSCs were mixed with Matrigel supplemented with VEGF-A (500 ng/mL) and implanted into the dorsal flank of Tie2-GFP mouse. Implanted Matrigel was immunostained for CD31+ blood vessels and stained for DAPI+ nuclei. Donor-derived CD31+/GFP− blood vessels are formed in the gel, whereas invasion of recipient-derived CD31+/GFP+ blood vessels into the gel is detected. White arrows indicate the implanted VYI-ECs that have been integrated into recipient vessel. Scale bars represent 50 μm.
Discussion
Neovascularization is a fundamental process that is essential not only for the development and maintenance of every organ, but also for the regeneration of failing organs. ECs derived from bone marrow, umbilical cord blood, and adipose tissue have been studied and applied for therapeutic neovascularization,36 and we have previously shown that freshly isolated donor stromal vascular fraction from adipose tissue can readily provide ECs that create vascular networks through “disassembly and reassembly” process, which can establish functional communication with the recipient circulation.35 However, obtaining a sufficient amount of ECs from adult organ is hindered because of the low expansion capacity of the ECs.
Our 2D Matrigel system is a simple, versatile method that does not require feeder cells for differentiating Flk1+ MPCs into several lineage precursor cells. Taking these advantages, we were able to dissect the downstream signaling pathways that underlie the VEGF-A–induced differentiation of ECs. In agreement with previous findings,8,11 we have confirmed PKA and PI3-kinase as the major downstream signaling pathways in the VEGF-A–induced differentiation and expansion of ECs. Meanwhile, we also observed that suppression of ROCK via Y27632 promotes the VEGF-A–induced differentiation and expansion of ECs by ∼ 4.2-fold, whereas the same profoundly suppressed the differentiation and expansion of α-SMA+ MCs. Given that the Y27632-induced differentiation and expansion of ECs could be recapitulated through other ROCK inhibitors and siROCK in various ESC cell lines and iPSCs, this ROCK suppression approach has a wide range of applicability. In addition, the simplicity of supplementing a small chemical compound Y27632 adds yet37 another advantage to this protocol for procuring ample amount of ECs from ESCs or iPSCs for therapeutic neovascularization.
We further investigated how ROCK suppression via Y27632 is able to promote the differentiation and expansion of ECs from Flk1+ MPCs. Our analyses revealed that the expansion of differentiated ECs by ROCK suppression was mainly derived from the combinative promotion of proliferation and adhesion of CD144+ ECs, rather than the suppression of apoptosis and anoikis of CD144+ ECs. In various types of adult ECs, including HUVECs, activation of PI3-kinase/Akt signaling is mainly involved in cellular survival,37 whereas activation of ERK is mainly involved in cellular proliferation.38 However, Y27632 did not activate ERK in the ECs in any condition, suggesting that the expansion of ECs did not result from ERK activation. Given that Y27632 activated Akt in the ECs, presumably through inactivation of PTEN,33 and that blockade of PI3-kinase prevented the Y27632-induced differentiation and expansion of ECs, PI3-kinase/Akt signaling seems to be the major pathway in the proliferation of ECs derived from Flk1+ MPCs. Collectively, simultaneous VEGF-A– and ROCK suppression-induced Akt activation seems to contribute to the promotion of differentiation and expansion of ECs.
During embryonic development, ECs are further differentiated and specified into arterial, venous, and lymphatic ECs by various molecular regulations, and each specified EC displays diversity and heterogeneity at the molecular level, which are represented by respective surface markers.5,6,39,40 Our phenotype analyses revealed that the ECs differentiated and expanded through VEGF-A stimulation and ROCK suppression faithfully expressed most pan-EC surface makers (CD31, CD144, Flk1, Tie2, endoglin, and ICAM2). Moreover, considering the fact that dll4, notch1, and claudin-5 were highly up-regulated in these cells, it can be said that these ECs were further differentiated toward arterial ECs.5,39,40
To maximize the number of ECs obtained from ESCs, we expanded the ICAM2+ ECs for 2 days using the same system supplemented with VEGF-A and Y27632. Indeed, not only did Y27632 promote adhesion ICAM2+ ECs onto the plate, but it also further expanded the ECs with VEGF-A through simultaneous promotion of cell proliferation and suppression of cell apoptosis. These ECs, which we referred to as VYI-ECs, showed that they had neovasculogenic potential in both in vitro and in vivo analyses. Furthermore, ROCK suppression positively promoted the neovasculogenic activities of VYI-ECs under VEGF-A stimulation, which is consistent with previous findings demonstrated in other EC types,41 but not with some reports,42-44 which could be the result of differences in experimental designs or cell types.
Although our results show that VYI-ECs do have in vivo neovasculogenic capabilities, the following technologies need to be developed to amplify the applicability of ESC-derived ECs for therapeutic neovascularization. First, advanced methods for synchronous differentiation and fate determination of ECs need to be developed. From this study, we realized that, even though CD31 and CD144 are commonly used as EC surface markers, they cannot serve as reliable markers for measuring the degree of EC differentiation. Unfortunately, no specific and reliable surface markers currently exist for measuring the degree of EC differentiation. Whereas fully differentiated ECs are able to form a mature and stable blood vessel network via typical vasculogenesis, partially differentiated ECs form an immature and unstable blood vessel network,45 and often are dedifferentiated into mesodermal cells when implanted in vivo, which would diminish their therapeutic effect as a whole. Second, more innovative methods for maintenance and further expansion of VYI-ECs need to be developed. We observed that VYI-ECs gradually went through senescence or apoptosis after they were fully confluent; and although cocktail of growth factors could partially delay such shortcomings, it is not enough to completely block the detrimental process. Methods such as 3-dimensional culture and addition of biomechanical stimuli could be helpful in overcoming these limitations and for establishing a reliable protocol for generating ECs suitable for application in therapeutic neovascularization.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sujin Seo and Eun Soon Lee for their technical assistance.
This work was supported by the National Research Foundation of Korea (grant 2011-0019268) funded by the MEST, Korea.
Authorship
Contribution: H.J.J., D.-K.C., S.-H.L., S.S., and J.-S.P. designed and performed the experiments and analyzed the data; H.J.J., J.S.L., and G.Y.K. generated the figures and wrote the manuscript; and J.H.S., D.-S.L., I.K., K.-C.H., and G.Y.K. designed and supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gou Young Koh, Graduate School of Medical Science and Engineering, Korea Advanced Institute of Science and Technology, 373-1, Guseong-dong, Daejeon, 305-701, Republic of Korea; e-mail: gykoh@kaist.ac.kr.
References
Author notes
H.J.J. and D.-K.C. contributed equally to this study.