To the editor:
Dabigatran is a new oral direct thrombin inhibitor approved to prevent stroke in atrial fibrillation. It has no antidote, and management of severe bleeding is uncertain. A participant in RE-LY (randomized trial that compared dabigatran with warfarin1 ) unintentionally underwent cardiac surgery with therapeutic dabigatran levels, resulting in massive postoperative bleeding. We report the timeline of bleeding, hemostatic parameters, and dabigatran plasma levels (by HPLC) in response to emergency management with rFVIIa and hemodialysis.
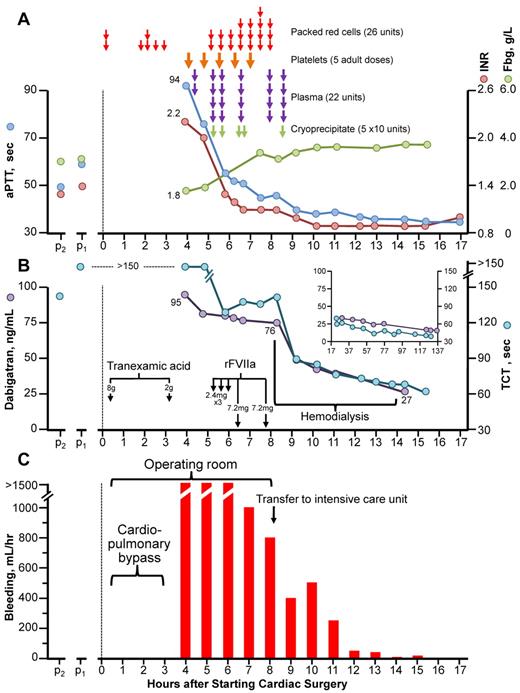
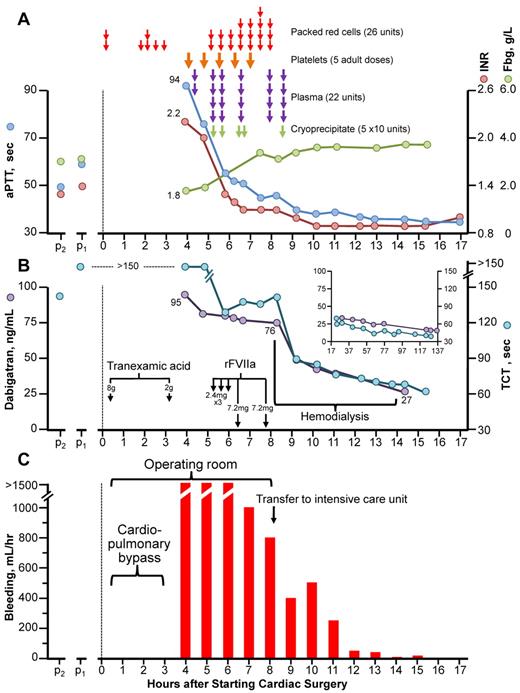
Figure 1 summarizes blood product administration, coagulation parameters (aPTT, INR, fibrinogen, thrombin-clotting time [TCT]), dabigatran levels, and bleeding.
Clinical course of dabigatran-associated massive postcardiac surgery bleeding. (A) Blood products administered, aPTT, INR, and fibrinogen (Fbg) over time. (B) Thrombin-clotting time (TCT) and plasma dabigatran levels. The inset shows TCT and dabigatran levels during the 17- to 137-hour postoperative period. Note: all TCT values shown did not decrease on in vitro addition of protamine (not shown), thus ruling out heparin as an explanation for the patient's prolonged TCTs. (C) Bleeding (chest/pericardial tube output/hr). p2 and p1 refer to blood samples obtained during stable dabigatran dosing 2 and 1 month, respectively, before surgery. rFVIIa indicates recombinant factor VIIa.
Clinical course of dabigatran-associated massive postcardiac surgery bleeding. (A) Blood products administered, aPTT, INR, and fibrinogen (Fbg) over time. (B) Thrombin-clotting time (TCT) and plasma dabigatran levels. The inset shows TCT and dabigatran levels during the 17- to 137-hour postoperative period. Note: all TCT values shown did not decrease on in vitro addition of protamine (not shown), thus ruling out heparin as an explanation for the patient's prolonged TCTs. (C) Bleeding (chest/pericardial tube output/hr). p2 and p1 refer to blood samples obtained during stable dabigatran dosing 2 and 1 month, respectively, before surgery. rFVIIa indicates recombinant factor VIIa.
A 79-year-old, 80-kg male with non–insulin-dependent diabetes and chronic renal insufficiency (estimated creatinine clearance, 36 mL/min) was taking dabigatran 150 mg twice-daily. Two blood samples taken on chronic dabigatran therapy (obtained 1 and 2 months preoperatively) showed mild INR prolongation (1.3, 1.4 [normal, 0.8-1.2]), increased aPTT (49, 60 seconds [normal, 22-35 seconds), prolonged TCT (128, > 150 seconds [normal, 20-30 seconds]), and normal fibrinogen (3.0, 3.1 g/L [normal, 1.6-4.2 g/L). Dabigatran was discontinued 2 days (4 doses) before surgery.
The patient underwent tissue aortic valve replacement and single-vessel coronary artery bypass grafting using cardiopulmonary bypass performed with standard heparin anticoagulation (35 000 units of heparin; postoperative reversal with 400 mg of protamine); 8 g of tranexamic acid was given preoperatively for routine bleeding prophylaxis. Postoperatively, severe bleeding (> 1500 mL/hr) persisted despite additional tranexamic acid (2 g) and protamine (200 mg), cryoprecipitate, plasma, and platelet transfusions (raising the platelet count from 86 to 131 × 109/L), preventing sternal closure. Three “cardiac” doses of rFVIIa (2.4 mg/dose) did not control the bleeding; at this time, testing showed INR = 1.2, PTT = 52, TCT = 129 (without in vitro correction by protamine); the platelet count was 127 × 109/L. After 2 “hemophiliac” doses of rFVIIa (7.2 mg/dose), bleeding fell to ∼ 800 mL/hr and the patient could be transferred to ICU for hemodialysis.
The patient was dialyzed (without addition of anticoagulant) for 6 hours with a high-flux dialysis filter (Polyflux 210h; Gambro). Dialysate flow was 700 mL/min; the average blood flow was 320 mL/min with 2 L net ultrafiltration. During hemodialysis, the bleeding subsided further.
The first plasma dabigatran level (40 minutes after surgery, and after 3 liters of blood product/crystalloid) was 95 ng/mL—a concentration typically seen on dabigatran in the RE-LY study.2 Immediately before hemodialysis, the dabigatran level was 76 ng/mL, and after 6 hours of hemodialysis was 27 ng/mL. No additional blood products were required. The sternum was closed 4 days later.
The postoperative course was complicated by prolonged ventilation/Enterobacter pneumonia, asymptomatic nonocclusive femoral DVT (by surveillance ultrasonography [postoperative day (POD 7)]), and acute-on-chronic renal failure. Discharge to a rehabilitation facility occurred on POD56.
This patient developed massive postoperative bleeding resulting from elective cardiac surgery performed with therapeutic dabigatran levels. This illustrates the importance of adjusting the number of days “off” dabigatran before surgery according to current renal function. Based on experience with this case and other information, it is now recommended3 for a patient with creatinine clearance 30-50 mL/min that dabigatran be stopped 4 days before elective major surgery, and that a normal (or near-normal) TCT be documented presurgery. In our patient, the TCTs (measured postoperatively) were all greatly elevated, and preoperative measurement would have avoided surgery. We observed an excellent correlation between the TCT and dabigatran levels in our patient (Figure 1B).
The case also provides support for the use of high-dose rFVIIa in reducing dabigatran-associated bleeding, as suggested by an animal study.4 In our case, hemodialysis reduced dabigatran levels quickly, consistent with a previous study.5 The combination of high-dose rFVIIa followed by hemodialysis helped to manage this life-threatening bleeding episode.
Authorship
Contribution: T.E.W., p.m., A.L., and C.R. provided patient care and performed first-hand clinical observations; T.E.W. and J.W.E. obtained laboratory data; and all of the authors reviewed the data and wrote the manuscript.
Conflict-of-interest disclosure: S.J.C. was principal investigator for the RE-LY trial; and J.W.E. reports receiving honoraria and research support from Boehringer Ingelheim, the company that developed and markets dabigatran etexilate. The remaining authors declare no competing financial interests.
Correspondence: Theodore E. Warkentin, Hamilton Health Sciences Centre, Hamilton General Site, 237 Barton Street East, Hamilton, ON L8L 2X2 Canada; e-mail: twarken@mcmaster.ca.