Abstract
The purine analogs, pentostatin and cladribine, induce high remission rates when used as first-line monotherapy for hairy cell leukemia (HCL); however, patients continue to relapse. Re-treatment with the same or alternate purine analog produces lower response rates and a shorter duration of response. Fludarabine is another purine analog widely used in indolent lymphoid cancers, often in combination with rituximab, but there are few reports of its use in HCL. We identified 15 patients treated in British Columbia with fludarabine and rituximab (FR) from 2004 to 2010 for relapsed/refractory HCL after first-line cladribine (n = 3) or after multiple lines of therapy (n = 12). All patients with available response data responded to FR. With median follow-up of 35 months, 14 patients remain progression-free, whereas 1 patient has developed progressive leukemia and died. Five-year progression-free and overall survivals are 89% and 83%, respectively. FR is a safe and effective therapeutic option for relapsed/refractory HCL.
Introduction
Despite marked improvements in therapy for hairy cell leukemia (HCL) since the introduction of purine analogs,1–4 30% to 40% of patients relapse.5,6 Progression-free survival (PFS) curves do not plateau, implying that the leukemia is not cured.7 Retreatment with cladribine or pentostatin leads to declining complete response (CR) rates and shorter PFS with each successive course.5–7 There is therefore a need for new, safe, effective therapies at the time of relapse.
Prior small studies examining chemoimmunotherapy either to eradicate minimal residual disease (MRD)8,9 or for relapsed/refractory HCL10 have used combinations of cladribine or pentostatin with rituximab. Fludarabine is another attractive option because of its mechanism of action, ability to administer multiple courses, convenient oral bioavailability, and well-understood spectrum of toxicity. There are only 2 case reports describing fludarabine use in HCL11,12 despite its widespread use in chronic lymphocytic leukemia and indolent non-Hodgkin lymphomas.13–16 We and others have previously demonstrated excellent overall survival, PFS, and tolerability with fludarabine and rituximab (FR) as initial therapy for chronic lymphocytic leukemia.17–19 Since 2004, we have recommended FR for patients with relapsed/refractory HCL and herein review our experience to assess the tolerability and efficacy of FR for such patients.
Methods
The British Columbia Cancer Agency (BCCA) Lymphoid Cancer Database was searched to identify all patients treated with FR for relapsed/refractory HCL. HCL was diagnosed according to World Health Organization criteria20 after central review to exclude HCL variant and other lymphocytic leukemias. We obtained clinical and follow-up information from hospital, clinical, and individual physicians' records. This study was approved by the University of British Columbia, BCCA Research Ethics Board.
Treatment
Treatment was reserved until symptomatic or threatening progression of HCL that was refractory to or relapsed after prior therapy. In practice, treatment was initiated at onset of neutropenia, thrombocytopenia, or anemia resulting in increased risk of infection or bleeding, symptomatic anemia, or symptomatic splenomegaly. The FR regimen consisted of fludarabine 40 mg/m2 per day orally on days 1 to 5 adjusted for renal function (50% dose reduction for creatinine clearance 30-70 mL/min; not used if creatinine clearance < 30 mL/min) and rituximab 375 mg/m2 intravenously on day 1 administered every 28 days for 4 cycles. No routine antibiotics or anti-virals were administered.
Response
Because FR treatment was for relapsed/refractory disease and we did not think sufficient additional benefit would accrue after delivery of the planned 4 cycles to offset the risks imposed by additional chemoimmunotherapy, no further treatment was planned unless symptomatic or threatening disease returned. Having decided that treatment would stop, regardless of depth of response, we did not undertake formal characterization of response with bone marrow (BM) or MRD assessments routinely. Response definitions are therefore as follows: unconfirmed complete response, morphologic absence of hairy cells in peripheral blood with complete normalization of peripheral blood counts and resolution of physical examination evidence of HCL; CR, all the above plus morphologic absence of hairy cells on BM biopsy; and partial response, greater than 50% improvement in all the above without meeting criteria for CR/unconfirmed complete response. When evaluated, MRD was assessed by morphology and immunophenotyping of peripheral blood or BM aspirate and immunohistochemistry of the BM biopsy.
Statistics
Overall survival was calculated from the date of initiation of FR to date of last follow-up or death from any cause. PFS was calculated from the date of treatment initiation to date of symptomatic progression requiring further therapy or death; patients alive without further progression were censored on the date of last follow-up. Data were analyzed using the Statistical Software Package for the Social Sciences (Version 14.0 for Windows).
Results and discussion
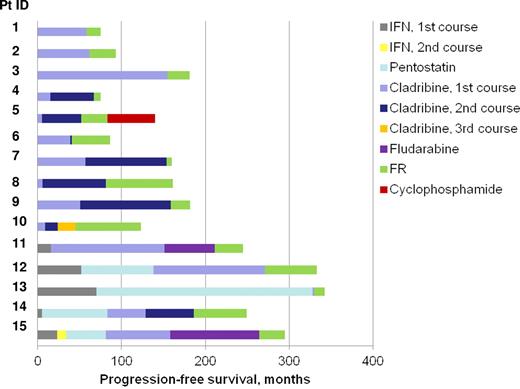
Of 254 HCL patients in our database, 28 developed symptomatic relapse of their HCL between January 2004 and September 2010. At their treating hematologist's discretion, 11 received cladribine, one received fludarabine, and one was too frail to permit treatment. Fifteen patients with relapsed (n = 13) or refractory (n = 2) disease were treated with FR after first-line cladribine (n = 2) or after multiple lines of therapy (n = 13). Patient characteristics and outcomes are shown in Table 1. Median age at FR was 59 years (range, 41-82 years). Patients were heavily pretreated with a median of 2 prior systemic treatments (range, 1-5). All patients had received at least one course of cladribine; 7 had received 2 courses (47%). A purine analog was the treatment immediately before FR in all patients: cladribine in 13 (87%) and fludarabine alone in 2 (14%). PFS for all therapies is shown in Figure 1.
Progression-free survival (PFS) by therapy. FR shown in green. One patient's leukemia progressed after therapy with FR (patient 5); the PFS for all other patients after FR represents the time to last follow-up in months.
Progression-free survival (PFS) by therapy. FR shown in green. One patient's leukemia progressed after therapy with FR (patient 5); the PFS for all other patients after FR represents the time to last follow-up in months.
FR therapy was administered at median 12.8 years from diagnosis (range, 3.4-27.5 years) and at median 60 months from the last therapy (range, 2-155 months). Patients underwent a median of 4 cycles of FR (range, 2-4 cycles). Oral fludarabine was well tolerated; no patient required intravenous formulation. Treatment was discontinued in one patient after 2 cycles for a hypersensitivity reaction to rituximab; fludarabine was discontinued in one patient after 3 cycles and development of interstitial lung disease. This latter patient received 6 rituximab infusions. Herpes zoster occurred in 2 patients: 1 during and one 6 months after FR. The latter patient had neutropenia at the time of infection with a normal BM biopsy. No patient required hospitalization during FR therapy.
Response data were available for 13 patients, although depth of response was not routinely assessed (Table 1). All 13 patients achieved normalization of peripheral blood counts, absence of circulating hairy cells, and resolution of splenomegaly if initially present. Three patients had BM biopsies at completion of therapy documenting MRD negativity. At median follow-up of 35 months from FR (range, 10-80 months), 14 patients remain progression-free without further therapy, whereas one patient developed recurrent disease 31 months after FR. For the whole cohort, 5-year PFS and overall survival are 89% and 83%, respectively.
Median follow-up for our cohort is 35 months, which is modest considering the long duration of response seen after purine analog therapy for HCL, even in the relapsed setting. However, 5 patients (patients 6, 8, 10, 13, and 14) have already had a longer duration of response with FR than with their previous line of therapy, including both patients with refractory disease (< 3→57, < 3→12, 21→78, 57→65, and 75→80 months), and all other remissions are ongoing (10, 13, 23, 26, 29, 33, 35, 39, and 66 months) except one (Figure 1). Patient 5, whose leukemia progressed 31 months after FR treatment, had a duration of response of 5 and 47 months with 2 prior cladribine treatments. The 5-year recurrence rate in our cohort was 7% (0 for those who received FR as second-line therapy, 7% for third- to sixth-line therapy). Else et al treated 8 patients with cladribine or pentostatin with rituximab for relapsed HCL and demonstrated a 2-year recurrence rate of 0% when administered as second-line therapy and 25% when administered as third- to fourth-line therapy.10 In a larger historical cohort from the Royal Mardsen Hospital, the 2-year recurrence rate after single-agent purine analog therapy was 15% for second-line therapy and 33% for third-line therapy.10 FR therefore appears at least comparable with other second-line treatments for relapsed/refractory HCL.
There are ongoing questions regarding the appropriate schedule of chemoimmunotherapy in HCL. Previous studies with cladribine or pentostatin used 3 to 8 doses of either sequential or concurrent rituximab.10 Other groups have used rituximab for 4 to 8 doses for eradication of MRD after initial therapy with cladribine.8,9 Byrd et al compared both concurrent and sequential FR administration as upfront therapy for chronic lymphocytic leukemia and found a superior overall response rate in the concurrent arm (90% vs 77%).18,21 A National Cancer Institute trial is examining assignment to either simultaneous cladribine and rituximab or to these 2 agents given in series.22
In conclusion, in this multiply relapsed and heavily pretreated group of patients with HCL, chemoimmunotherapy with oral FR was well tolerated, safe, and effective. Reflecting the rarity of HCL, this is one of the largest series examining chemoimmunotherapy in HCL to date. FR may be given successfully to community-based patients for relapsed or refractory HCL, including those previously exposed to alternate purine analogs. Prospective clinical trials examining chemoimmunotherapy for relapsed HCL will be vital in determining the optimal treatment for such patients.
Presented in abstract form at the 52nd annual meeting of the American Society of Hematology, Orlando, FL, December 5, 2010.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The BCCA Lymphoid Cancer Database is supported in part by contributions of the Turner Family Fund and the Mary Toye Fund.
Authorship
Contribution: A.S.G. and J.M.C. designed and performed research, collected data, and analyzed data; L.N.Z. performed research and collected data; and all authors wrote the manuscript.
Conflict-of-interest disclosure: The BCCA (J.M.C.) has received research funding from F Hoffmann-La Roche (Roche Canada). The remaining authors declare no competing financial interests.
Correspondence: Alina S. Gerrie, Division of Hematology, University of British Columbia, 2775 Laurel St, Vancouver, BC, Canada V5Z 1M9; e-mail: agerrie@bccancer.bc.ca.