To the editor:
In their recent paper, Zhang and collaborators claim that they have identified a stimulatory role of soluble guanylyl cyclase (sGC) in platelets using platelet-specific sGC-deficient mice.1 These data are in contrast to numerous studies by many different investigators in the field of sGC research and to our recent publications. We studied both a global sGC knockout (KO) mouse2 and exactly the same platelet-specific sGC KO3 mouse model as that reported by Zhang et al.1 In our, and many other studies, it is clearly demonstrated that the NO/sGC/cGMP/PKG pathway plays an exclusively inhibitory role in platelets. We and others have never obtained evidence for any “stimulatory” role of sGC in platelets.
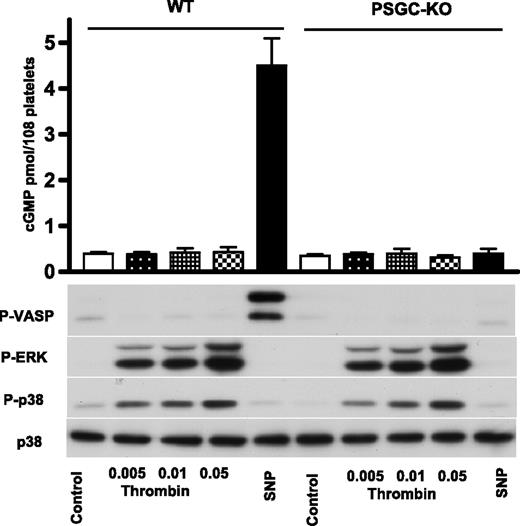
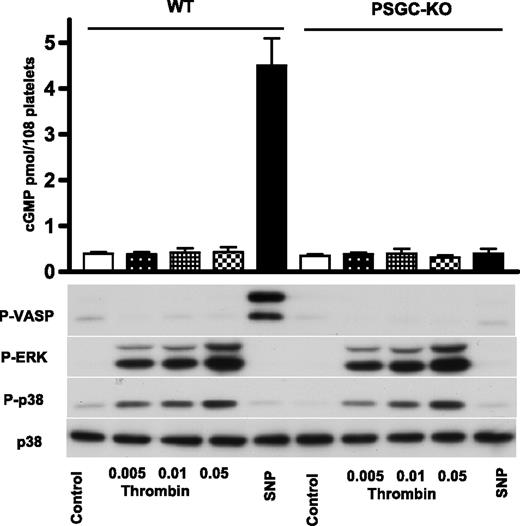
In the article by Zhang and collaborators, the key experiment presented in Figure 2A shows that thrombin increases platelet cGMP content, and proposes a “stimulatory” role of sGC in platelet activation.1 First, these data are not consistent with the previous data from the same group.4,5 In Marjanovich et al (Figure 74 ) the same thrombin concentration (0.05 U/mL, 5 minutes) increased cGMP content in WT mouse platelets only 3-fold (from 300 to 900 fmol/108 platelets), whereas in Zhang et al,1 it increased the cGMP content 13-fold (from 100 to 1300 pmol/108 platelets), which is comparable with the cGMP increase induced by 10μM sodium nitroprusside (SNP; Figure 1D1 ). Second, if these data are correct, then the authors should also be able to show sGC/cGMP-induced VASP phosphorylation induced by thrombin stimulation. However, in all 3 of the papers mentioned above, the authors did not present any data on sGC/cGMP-induced VASP phosphorylation. Therefore, we decided to reproduce exactly this key experiment using the same platelet-specific sGC KO mouse model. Moreover, we used 3 different methods for cGMP determination (2 ELISAs, including one from the same supplier used by Zhang et al,1 and a cGMP RIA). The data in Figure 1 summarize the changes in cGMP content and VASP phosphorylation in platelets induced by thrombin and SNP in WT and platelet-specific sGC KO mouse platelets. Our experiments clearly show no detectable thrombin-induced increase in cGMP; moreover, VASP phosphorylation is even slightly decreased in thrombin-stimulated platelets (Figure 1).
Thrombin neither activates platelet sGC nor induces VASP phosphorylation. Platelets from wild type (WT) and platelet-specific sGC KO mice (PSGC-KO) were isolated exactly according to the protocol used by Zhang et al1 and incubated with the indicated concentrations of thrombin (5 minutes) or 10μM of SNP (1 minute). Parts of the samples were used for cGMP measurement and the rest for Western blot analysis of VASP, p38, and ERK phosphorylation. Results are representative of 5 independent experiments.
Thrombin neither activates platelet sGC nor induces VASP phosphorylation. Platelets from wild type (WT) and platelet-specific sGC KO mice (PSGC-KO) were isolated exactly according to the protocol used by Zhang et al1 and incubated with the indicated concentrations of thrombin (5 minutes) or 10μM of SNP (1 minute). Parts of the samples were used for cGMP measurement and the rest for Western blot analysis of VASP, p38, and ERK phosphorylation. Results are representative of 5 independent experiments.
In their discussion, Zhang et al state that the 2 major findings in their recent study are: first, that sGC plays a stimulatory role in platelet activation.1 This does not agree with several other studies as mentioned previously. Second, they reported that millimolar concentrations of SNP can mediate sGC-independent platelet inhibition. This was already described for human platelets by Marcondes et al in 2006,6 which was even cited by Zhang et al1 (reference 43 in Zhang et al), but for other reasons.
In summary, in our experiments we show that thrombin neither increases cGMP nor the subsequent cGMP-dependent VASP phosphorylation in platelets. Our data and those of many other groups do not support a “sGC platelet stimulatory concept.” In contrast, our present data and that of many other authors confirm the concept that the NO/sGC/cGMP/PKG pathway plays exclusively inhibitory roles in platelets.
Authorship
Contribution: S.G. and A.F. performed the research and analyzed the data; and S.G. and U.W. wrote the final version of the letter.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stepan Gambaryan, Institut für Klinische Biochemie & Pathobiochemie, University of Wuerzburg, Grombuehlstr 12, D-97080 Wuerzburg, Germany; e-mail: gambaryan@klin-biochem.uni-wuerzburg.de; or Ulrich Walter, Centrum for Thrombosis and Hemostasis, University of Mainz, D-55131 Mainz, Germany; e-mail: ulrich.walter@unimedizib-mainz.de.