Abstract
In 2008, the World Health Organization introduced CEBPA (encoding the CCAAT/enhancer binding protein)–mutated acute myeloid leukemia (AML) as a provisional entity. However, the classification of CEBPA-mutated AML with multilineage dysplasia (MLD; ≥ 50% dysplastic cells in 2-3 lineages) remains to be clarified. In the present study, we investigated 108 CEBPA-mutated AML patients for the impact of MLD, karyotype, and additional mutations. MLD+ patients differed from MLD− patients only by lower mean WBC counts, not by biologic characteristics, cytogenetic risk profiles, or additional mutations. Survival was better for female patients, patients < 60 years of age, for intermediate versus adverse karyotypes, and, in the case of FLT3-ITD negativity, biallelic versus monoallelic/homozygous CEBPA mutations. In contrast, 2-year overall survival and event-free survival did not differ significantly between MLD+ and MLD− patients. By univariable Cox regression analysis, sex, age, WBC count, and cytogenetic risk category were related to overall survival, but MLD was not. Therefore, because dysplasia is not relevant for this subtype, CEBPA-mutated AML patients should be characterized only according to mutation status, cytogenetic risk group, or additional mutations.
Introduction
Intragenetic mutations of CEBPA (encoding the CCAAT/enhancer binding protein) exist in 7%-15% of normal karyotype acute myeloid leukemia (AML) patients and are favorable at least as biallelic mutations.1-5 In normal karyotype AML, Dufour et al described improved survival outcomes for biallelic CEBPA-mutated patients compared with CEBPA-wild-type, whereas monoallelic mutations and CEBPA-wild-type had similar outcomes.4 The prognostic benefit is lost in cases of coincidence with FLT3-ITD3,6 or DNMT3A mutations.5 Renneville et al documented improved prognosis only for CEBPA-mutated patients with normal karyotypes and without FLT3-ITD compared with corresponding CEBPA wild-type patients.6 Schnittger et al observed improved survival for biallelic CEBPA mutations compared with monoallelic/homozygous mutations.7 AML with mutated CEBPA or NPM18 received the status of new provisional entities within the World Health Organization (WHO) category “AML with recurrent genetic abnormalities,”9 but their status as disease entities rather than as prognostic factors has to be confirmed.9 Patients without “recurrent genetic abnormalities” are classified as “AML with MDS-related changes, AML-MRC” in cases of a myelodysplastic syndrome (MDS)–related cytogenetic abnormality, a previous myeloid malignancy, or presence of multilineage dysplasia (MLD) with dysplastic features in ≥ 50% of cells in ≥ 2 hematopoietic lineages.10 It remains unclear how to classify AML patients with MLD without prior MDS or MDS-related cytogenetic abnormalities and with a CEBPA or NPM1 mutation. The WHO recommends classifying them as “AML with myelodysplasia-related changes” and to mention in addition the respective mutation (CEBPA or NPM1).10 To investigate the biologic justification of this separate entity, we analyzed 108 CEBPA-mutated AML patients for the prognostic impact of MLD.
Methods
In the present study, we analyzed 108 patients at diagnosis of CEBPA-mutated AML all evaluable for MLD (54 male and 54 female patients; median age, 67.2 years; range, 15.7-87.6 years) between August 2005 and June 2011. Most patients (n = 99 [91.7%]) had de novo AML (s-AML, n = 6 [5.6%]; t-AML, n = 3 [2.8%]). Patients were treated according to standard AML protocols, including “7 + 3” or combinations of chemotherapeutics such as TAD and HAM.11 BM samples (in part combined with peripheral blood) were sent from different hematologic centers to the MLL Munich Leukemia Laboratory. All patients gave written informed consent for research studies. The study was approved by the Internal Review Board and followed the Declaration of Helsinki.
All samples underwent May-Grünwald-Giemsa staining and cytochemistry (myeloperoxidase and nonspecific esterase).12 Two hundred nucleated cells were investigated. Dysplasia was assessed in granulopoiesis, erythropoiesis, and megakaryopoiesis according to Goasguen et al and WHO criteria.10,13 MLD was defined by ≥ 50% dysplastic cells in 2-3 lineages following the WHO guidelines.10 In 18 of 108 patients, only 2 hematopoietic lineages were evaluable, but patients could be defined as MLD+ in cases of 2 dysplastic lineages or as MLD− if 2 lineages were without dysplasia. Cytomorphology was done by 2 investigators (K.M. and U.B.) and all cases were reviewed by 1 investigator (T.H.). Cytogenetics (in all patients), investigation of additional mutations (in large subsets of patients), and gene-expression analysis (in some of the patients) are described in supplemental Tables and Figures (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Raw data are available at the Gene Expression Omnibus under accession number GSE33223 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc = GSE33223).
Results and discussion
There was a preponderance of M1 and M2 FAB14,15 subtypes (M0, n = 1; M1, n = 52; M2, n = 47; M4, n = 7; and M6, n = 1). Dysplasia (≥ 50%) was detected in granulopoiesis in 46 of 106 patients (43.4%), in erythropoiesis in 14 of 108 (13.0%), and in megakaryopoiesis in 34 of 90 (37.8%). A total of 44 patients (40.7%) had no dysplastic cell lineage, 36 (33.3%) had unilineage dysplasia (≥ 50%), 26 (24.1%) had bilineage dysplasia, and only 2 (1.9%) had trilineage dysplasia. Therefore, MLD according to WHO standards (≥ 50% of dysplastic cells in ≥ 2 lineages)10 was found in 28 of 108 (25.9%) BM samples. CEBPA mutations were biallelic in 54 of 108 (50.0%), monoallelic in 44 (40.7%), and homozygous in 10 (9.3%). Most frequent were: additional NPM1 mutations, 15 of 108 (13.9%); IDH1/2 mutations, 16 of 104 (15.4%); FLT3-ITD mutations, 10 of 105 (9.5%); RUNX1 mutations, 11 of 107 (10.3%); FLT3-TKD mutations, 4 of 108 (3.7%); and MLL-PTD mutations, 4 of 108 (3.7%). Eighty of 108 (74.1%) patients had normal karyotypes, 28 of 108 (25.9%) had cytogenetic alterations (−7, n = 6; +8 sole, n = 3; −Y, n = 2; other trisomies, n = 7; other unbalanced alterations, n = 9; balanced translocations, n = 1). According to Medical Research Council (MRC) criteria,16 the majority of patients had prognostically intermediate karyotypes (102 of 108 [94.4%]) mainly because of normal karyotypes. Six patients (5.6%) had adverse karyotypes (all with −7). MRC risk groups did not differ significantly between biallelic and monoallelic/homozygous CEBPA-mutated AML patients.
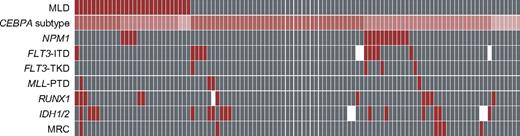
MLD+ patients differed by lower mean WBC counts from MLD− (P = .004), but other parameters (male/female ratio, mean age, platelet/hemoglobin levels, and percentage of BM blasts as continuous parameters) did not differ significantly. The distribution of biallelic (MLD+, 11 of 28 [39.3%]; MLD−, 43 of 80 [53.8%]), monoallelic (14 of 28 [50.0%] vs 30 of 80 [37.5%]), and homozygous (3 of 28 [10.7%] vs 7 of 80 [8.8%]). CEBPA mutations did not differ significantly between MLD+ and MLD− patients. Additional mutations were also similarly distributed between both cohorts (Figure 1 and supplemental Table 1). Additional NPM1 mutations were detected only in monoallelic CEBPA-mutated patients (frequency, 15 of 45 [33.3%] in this subgroup), but did not occur in biallelic CEBPA-mutated patients (P < .001). Other mutations investigated for this aspect (FLT3-ITD, IDH1/2, RUNX1) did not differ significantly between biallelic and monoallelic/homozygous CEBPA mutations. Intermediate karyotypes16 were identified in the majority of MLD+ patients (27 of 28 [96.4%]) and MLD− patients (75 of 80 [93.8%]). Adverse karyotypes were rare in both cohorts (MLD+ patients, 1 of 28 [3.6%]; MLD− patients, 5 of 80 [6.3%]; P = nonsignificant). Cytogenetic alterations did not differ significantly between MLD+ and MLD− patients (8 of 28 [28.6%] vs 20 of 80 [25.0%], respectively).
Frequency of cytogenetic alterations and of additional molecular mutations in MLD+ and MLD− patients. Red indicates a positive characteristic of the respective marker and gray indicates negativity. White cells show that the evaluation was not done for this patient. In the case of MRC,16 red shows the adverse-risk group and gray shows intermediate risk. Patients are depicted vertically. In cases of CEBPA subtype, dark red shows biallelic, medium red shows monoallelic, and bright red shows homozygous CEBPA mutations.
Frequency of cytogenetic alterations and of additional molecular mutations in MLD+ and MLD− patients. Red indicates a positive characteristic of the respective marker and gray indicates negativity. White cells show that the evaluation was not done for this patient. In the case of MRC,16 red shows the adverse-risk group and gray shows intermediate risk. Patients are depicted vertically. In cases of CEBPA subtype, dark red shows biallelic, medium red shows monoallelic, and bright red shows homozygous CEBPA mutations.
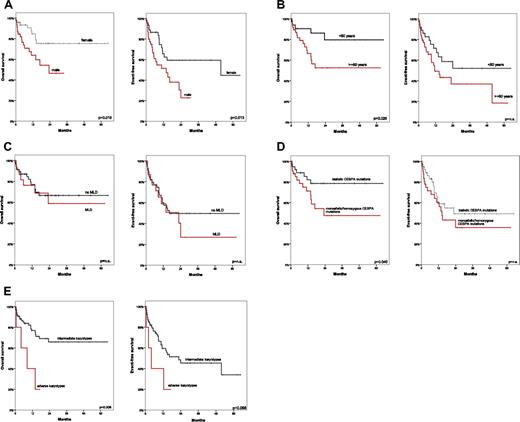
For the total cohort, median overall survival (OS) was not reached (2-year OS, 62.4%); the median event-free survival (EFS) was 16.3 months (2-year EFS, 46.4%). The median OS (P = .019) and EFS (P = .013) were better for female than male patients (Figure 2A). Patients < 60 years of age had better 2-year OS than those ≥ 60 (P = .026; Figure 2B). De novo AML versus s-AML/t-AML had no significant influence. When dysplasia (≥ 50%) was evaluated separately in granulopoiesis, erythropoiesis, or megakaryopoiesis independently from other lineages, median OS/EFS was independent of dysplasia. The presence of MLD did not affect survival outcomes significantly (2-year OS for MLD+ patients, 56.5%; 2-year OS for MLD− patients, 65.5%; 2-year-EFS, 38.8% vs 49.8%, respectively; P = nonsignificant; Figure 2C and supplemental Table 2). When only FLT3-ITD− patients were considered, biallelic mutations compared with monoallelic/homozygous CEBPA mutations had significantly better OS (median not reached vs 23.3 months; P = .040; Figure 2D). Additional mutations had no prognostic impact. Intermediate karyotypes had better OS than adverse karyotypes (median not reached vs 8.4 months; P = .006) and better EFS (22.8 vs 4.1 month; P = .068; Figure 2E).
Comparison of OS and EFS. Shown is a comparison of OS and EFS in female (A) versus male (B) patients ≥ 60 years of age versus < 60 years; in patients with multilineage dysplasia compared with those without MLD (C); in patients with biallelic versus monoallelic/homozygous CEBPA mutations in the FLT3-ITD− subgroup (D); and in patients with intermediate versus adverse karyotypes according to MRC criteria (E).16
Comparison of OS and EFS. Shown is a comparison of OS and EFS in female (A) versus male (B) patients ≥ 60 years of age versus < 60 years; in patients with multilineage dysplasia compared with those without MLD (C); in patients with biallelic versus monoallelic/homozygous CEBPA mutations in the FLT3-ITD− subgroup (D); and in patients with intermediate versus adverse karyotypes according to MRC criteria (E).16
By univariable Cox regression analysis for OS, younger age (P = .031), lower WBC count (P = .003), female sex (P = .024), and intermediate MRC risk category (P = .03) were favorable prognostically. The presence of MLD, hemoglobin/platelet level, percentage of BM blasts, biallelic versus monoallelic/homozygous CEBPA mutations, FLT3-ITD mutations, and NPM1 mutations were not significant. By multivariable Cox regression analysis for OS, female sex (P = .01), lower WBC count (P = .001), and intermediate MRC category (P = .045) remained favorable parameters. MLD did not affect EFS in univariable analysis (supplemental Table 3).
Gene-expression profiling confirmed the unique signature of biallelic CEBPA-mutated AML1,2 independent of MLD (supplemental Figures 1A-B and 2, and supplemental Table 4).
In conclusion, MLD+ and MLD−CEBPA-mutated AML do not differ in regard to biologic characteristics, AML history, CEBPA-mutation characteristics (biallelic vs monoallelic/homozygous), cytogenetic risk profiles,16 or additional mutations. MLD does not influence the prognosis of CEBPA-mutated AML, whereas biologic characteristics, cytogenetic risk group, and CEBPA-mutation characteristics are relevant prognostically. There is no prognostic impact of MLD in NPM1-mutated AML.17 Our results do not support the decision to overrule the detection of CEBPA mutations for classification and prognosis by the presence of MLD. We strongly support the classification of CEBPA-mutated AML as a separate entity, as suggested by the WHO,9 and the subclassification of CEBPA-mutated patients only according to mutation characteristics, cytogenetic risk profiles, and additional mutations.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all of the physicians for sending patient samples to the MLL Munich Leukemia Laboratory and for providing clinical information; the participating centers and investigators (contributing 4 or more patients) in order of the number of patients provided: Klinikum Augsburg (C. Schmid), Universitätsklinikum Köln (K.-A. Kreuzer), Städtisches Klinikum München Schwabing (C. Wendtner), Klinikum Fulda (H.-G. Höffkes), and Johanniter-Krankenhaus Bonn (Y. Ko); and our coworkers at the MLL Munich Leukemia Laboratory for their dedicated work.
Authorship
Contribution: U.B. analyzed the data and wrote the first draft of the manuscript; S.S. and V.G. performed the molecular analysis; U.B., K.M., and T.H. evaluated cytomorphology and dysplasia; V.G., A. Kohlmann, and A. Kowarsch performed gene-expression profiling and microarray data analysis; T.A., N.N., and W.K. performed the statistical analysis; C.H. evaluated the cytogenetics; T.H. designed the study; and all authors wrote, reviewed, and approved the final version of the manuscript.
Conflict-of-interest disclosure: S.S., W.K., C.H., and T.H. are part owners of the MLL Munich Leukemia Laboratory. K.M., V.G., A. Kohlmann, T.A., A. Kowarsch, and N.N. are employed by the MLL Munich Leukemia Laboratory. U.B. declares no competing financial interests.
Correspondence: Torsten Haferlach, MD, MLL Munich Leukemia Laboratory, Max-Lebsche-Platz 31, 81377 Munich, Germany; e-mail: torsten.haferlach@mll.com.