Abstract
Monoclonal B-cell lymphocytosis (MBL), a newly recognized entity found in approximately 3% of normal persons, precedes chronic lymphocytic leukemia. However, MBLs progress into overt malignancy only in a very minor portion of cases, thus raising the clinical concern of whether and how we can discriminate at diagnosis which rare cases will evolve into a fully fledged tumor. Understanding the molecular/biologic features underlying the risk of progression may significantly modify our strategies for correctly managing B-cell premalignant states. MBL cells bear the same chromosomal abnormalities of chronic lymphocytic leukemia. Genome-wide sequencing and animal models indicate that genetic abnormalities disrupting the control of cell growth and survival cooperate with microenvironment-triggered events, mainly represented by antigen-mediated B-cell receptor and coreceptor stimulation, to trigger and fuel clonal expansion. The initial functional activation of survival/proliferation pathways may later become subsidized by autonomous genetic abnormalities (eg, a single mutation) affecting the same or parallel critical signaling pathway(s).
Introduction
Mature B-cell tumors represent approximately 4% of new cancers arising each year in the world.1 B-cell premalignant states are vastly more common, but only rare cases progress into overt malignancy, raising the clinical concern of whether and how we can discriminate at diagnosis which cases will evolve into a fully fledged tumor. At diagnosis, we are presently unable to unequivocally state to the individual subject to which category (progressive or nonprogressive) he/she belongs. This causes frustration in the doctor and anxiety in the subject, both leading to controls and tests that are economically and socially costly. Understanding the molecular/biologic features associated with a risk of progression would significantly impact on the strategies of clinical management of B-cell premalignancies. This would allow physicians to focus onto the rare risky subjects and to refrain from unnecessary monitoring of the majority of otherwise healthy persons.
The presence of tiny numbers of apparently indolent monoclonal B cells with a malignancy-specific phenotype is common to the very early phases of most mature B-cell tumors. The time-honored example is monoclonal gammopathy of uncertain significance (MGUS), which has an incidence of 1% in the population older than 50 years (and up to 10% older than 75 years).2 At least 2 independent studies3,4 have demonstrated that most cases of multiple myeloma are preceded by MGUS whose transformation rate is 1% to 2% per year.5 A more recent example of B-cell premalignant state is the detection of in situ lymph node aggregations of cells that reproduce the immunophenotypic and molecular features of follicular lymphoma6,7 and/or of mantle cell lymphoma. These aggregations that appear to represent “in situ lymphomas” are found incidentally, show a localization restricted to the areas usually involved by follicular lymphoma or mantle cell lymphoma,8 and have an uncertain clinical behavior.
The entity defined monoclonal B-cell lymphocytosis (MBL)9 has given great impulse to investigate how B-cell premalignant states relate to overt malignancies.
MBL: terra incognita
MBLs are found in approximately 3% of normal persons and even attain higher frequency (> 10%) when more sophisticated analytical techniques are applied.10-13 Most cases are characterized by the presence of circulating monoclonal B cells, which have the phenotype of chronic lymphocytic leukemia (CLL-like MBL). MBLs are more common among males and in the advanced age (same sex and age predisposition as CLL), and their frequency is significantly increased (13.5%) in relatives of CLL patients in line with the well-known familial character of CLL.14,15 A large study has demonstrated that, as multiple myeloma is preceded by MGUS, CLL is always preceded by an MBL state.16 It has also been shown that MBL may progress into CLL with a frequency of 1% to 2% per year.17-19 That notwithstanding, as with MGUS, we do not have a clue as to whether individual subjects with MBL will or will not progress into fully fledged CLL or when this event will occur.
MBL is complex and heterogeneous, and it arises in a background of several B-cell subsets. First, circulating MBLs can be found whose phenotype differs from CLL (non–CLL-like MBL).11 These MBLs might result from the transient activation of the immune system by agents, such as those causing intercurrent infections.20 Still, the possibility exists that they may be premalignant states of other mature B-cell malignancies (eg, splenic marginal zone lymphoma) bearing similarities to “in situ lymphomas.”7,8 Next, 2 categories of CLL-like MBL exist.21 One is represented by clinical MBLs, as we may call the MBLs, which are detected in the context of lymphocytosis investigated with laboratory techniques.17 The second category is represented by the MBLs discovered while screening perfectly normal persons with the specific scientific purpose of identifying the presence of an MBL population.10 In what we might call population-screening MBLs, the absolute number of lymphocytes is not increased, rather a number of monoclonal B cells, in the range of few dozen monoclonal B cells per microliter, is detectable within a majority of polyclonal B lymphocytes.22 Further, some phenotypically population screening CLL-like MBLs are indeed polyclonal.10,23 Clearly, the suggestion is that, similar to the events occurring in other cancers, the development of CLL may start from a mixture of polyclonal cells with one clone progressively taking over.
Surprisingly, not only clinical MBLs,17,18 but also population-screening MBLs, bear the cytogenetic abnormalities, which are the hallmark of CLL, including 13q−, 17p−, and trisomy 12.12,20 In population-screening MBLs, these abnormalities are observed even when the number of circulating monoclonal CLL-like cells is extremely small and the subjects do not show any evidence of progression. This finding recalls the observation that in most MGUS monoclonal plasma cells frequently bear the same chromosomal abnormalities of multiple myeloma24,25 and that cells carrying the t14;18 translocation, the cytogenetic hallmark of follicular lymphoma, can be found in the peripheral blood of approximately 50% of healthy persons.26 Accordingly, it may be asked whether the investigation of MBL is pursuing the right track toward understanding the development of CLL.
The key question
The key question raised by these observations is whether, given enough time, all population-screening MBLs will become clinical MBLs and in turn every clinical MBL will become overt CLL: in other words, whether we have a time-dependent pipeline of unavoidable events or whether population-screening MBL may be simply considered an example of immune senescence while clinical MBLs are already CLL-committed. More realistically, the possibility has to be taken into account that clinical MBLs may be an offspring of rare population-screening MBLs progressing because of a random, individual (unpredictable?) event and similarly that each full-blown CLL is a rare, unfortunate descendant emerging from the large reservoir of clinical MBLs.
To this end, 2 related issues deserve mention. The first is whether the development of MBL is an inevitable fate for all human beings provided they live long enough. This appears to be a reasonable possibility considering that the MBL frequency increases with age becoming 50% to 75% in persons older 90 years of age.12,27,28 The second aspect to consider is whether given enough time every single CLL will progress from stage 0 to stage 4. Although this is the prevalent situation, there are certainly some stage 0 CLL patients who are absolutely stable during a period of more than 20 years.29 Their malignant cells have an incapacitated proliferative activity but a very efficient survival, not unlike the “tumor dormancy” observed in solid tumors. We might increase the IWCLL (International Workshop on CLL) allowable threshold guidelines to diagnose stage 0 CLL.30 This would allow some rare stage 0 patients who do not progress to be demoted into an MBL state (where they conceptually belong).31-33 That would perhaps allow a more proper classification of MBL and possibly reassure some rare patients. However, because clinically it would be retrospective, it would not lead to an understanding of which MBL will progress and which will not. To approach this problem, we have to integrate 2 different perspectives.
The genetic and microenvironment perspectives
If CLL-associated cytogenetic abnormalities are found already in population screening MBLs, we have to start reconsidering the actual pathogenetic role of the “classic” (cyto)genetic abnormalities. As the genetic architecture of CLL is progressively unraveled by microRNA studies and by deep-sequencing investigations, a new field of research is geared into action aimed at detecting novel genetic driving forces able to ignite the cellular proliferation that leads to clonal expansion. Are the rare cases that progress from MBL into CLL cancer-committed from the very beginning because of hitherto unknown mutations that affect critical gene function(s)? Or are they “corrupted” during their lifetime? How can we detect them? Is the risk of acquiring such (unknown) mutations maintained throughout the entire MBL life so that it will be impossible to ever stop worrying about progression?
The first attempts of genome-wide sequencing are identifying new relevant genes, such as MyD88,34 NOTCH1,34 and SF3B1,35,36 which are mutated in a variable, though essentially small, proportion of CLL cases. It is of interest that SF3B1 mutations occurs primarily in CLL with 11q−36 and that NOTCH1 mutations are associated with trisomy 12.37 In general, these recurrent mutations appear to be acting in the later stages of CLL, and some may underlie the Richter syndrome transformation.38 Conceivably, some mutations may be harbored by a subclone that will progressively take over. It is intriguing that, altogether, these abnormalities appear to be elucidating the genetic bases of CLL progression rather than of MBL evolution: the initiating genetic abnormalities remain unclear. The evidence may simply underline the necessity of different technical and experimental approaches able to unravel also the initiating lesions. However, one should also take into account the possibility that the role of genetic abnormalities may be very limited or even nonexisting in the early phases of MBL development, where microenvironmental stimuli could instead play a more prominent role.
Along the same reasoning, one has to consider that also CLL cells need a continuous support from the surrounding tissue microenvironment in terms of signaling pathways that favor clonal expansion, account for intraclonal complexity, and create a situation advantageous for the development of dangerous subclones.39 A critical component of the CLL microenvironment is antigen (Ag) stimulation through the B-cell antigen receptor (BCR).40 The striking degree of sequence similarity, observed in the BCRs of almost 30% of unrelated CLL cases regardless of the IGHV gene mutational status of the corresponding antibodies,41,42 has been taken to indicate the promoting pressure of a limited set of structurally similar antigenic epitopes. The dissection of the relevant epitopes indicates that molecular structures normally involved in eliminating cellular debris, scavenging apoptotic cells, and providing a first line of defense against pathogenic bacteria43-45 may trigger and/or facilitate the onset and evolution of at least some CLL clones. Similarly, evidence exist that the parallel stimulation of coreceptors such as CD40 and/or Toll-like receptors (TLRs) is probably relevant to obtain a full activation of leukemic B cells.46,47 These observations led us to conclude that Ag-induced activation may represent a key triggering event able to promote the development of MBL and to favor their progression into CLL by providing an ongoing proliferative stimulation.
Can animal models help solving the conundrum?
Recently, numerous animal models of CLL have been developed. Their analysis leads to consider 3 elements of main interest. The first is related to the TCL1-tg mouse model (the most studied in CLL).48 When TCL1 mice are crossed with mice knocked out for specific genes potentially relevant for CLL (examples being BAFF,49 HS1,50 and Tir846 ), the progeny of knockout/transgenic mice always show an evident acceleration of disease development. Therefore, these genes appear to actually influence the natural history of the disease; but at the same time, were all these genes concomitantly operating in patients, CLL should be a very aggressive disease in all cases and this is not the case.
The second element of interest is provided by a recent model where the deletion of the entire 13q14-minimal deleted region, which encodes the DLEU2/miR-15a/16-1 cluster,51,52 leads to the development of low penetrance indolent B-cell clonal lymphoproliferative disorders that appear to recapitulate the whole spectrum of human CLL-associated phenotypes, from MBL to Richter syndrome.53 Interestingly, a naturally occurring animal model, the New Zealand black mouse, has been shown to carry a point mutation in the same region of synteny, in particular in the 3′ flanking sequence of the identical microRNA, mir-16-1.54 In addition, in this model, the animals develop a monoclonal lymphoproliferative expansion characterized by increased numbers of CD5+ B220dull B cells, and the full disease appears only later during lifetime. The functional dissection of the 13q14 tumor suppressor locus provided by these models underlines the critical importance of the deleted region, which appears to harbor the gene(s) involved also in the first steps that lead to CLL. At the same time, it also indicates the importance of antigenic stimulation considering both the stereotypic gene usage by clonal cells55 and the long time of onset of each condition. The conclusion is that genetic abnormalities disrupting the control of cell growth and survival cooperate with antigenic stimulation to trigger and fuel clonal expansion. This conclusion is supported by xenograft models based on the transplantation of immunodeficient mice with human cells. A novel adoptive transfer model of human CLL in NOD/Sci-scid-IL2rg−/− mice gives strength to the idea already apparent from in vitro studies56 that autologous CD4+ T cells activated in vivo by alloantigens have a relevant role in promoting CLL cell survival and expansion.57
The third interesting aspect is the recent demonstration in a xenogeneic mouse model58 that HSCs from CLL patients have the propensity to generate clonal B cells (although the VDJ genes are always unrelated to those of the original CLL cells), suggesting that CLL-HSCs have intrinsic abnormalities that cause their skewing toward the B-cell lineage.
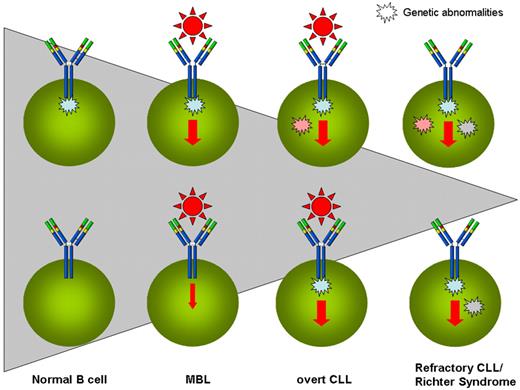
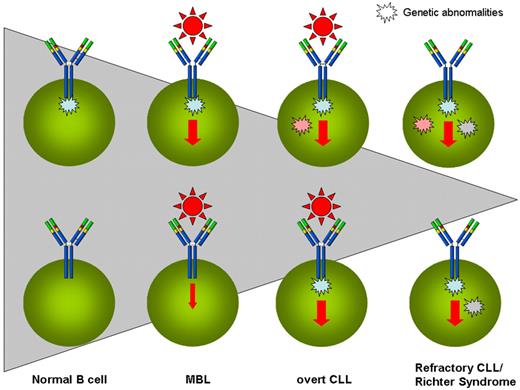
Taken together, animal models corroborate the scenario emerging in humans and suggest that critical genes are the instigators that pave the way toward the development of MBL/CLL and that ongoing antigenic stimulation through the BCR together with accessory cells operating within specific microenvironmental niches are the final executors (Figure 1).
The progressive evolution from antigen (Ag) stimulation to molecular abnormalities in the natural history of CLL. MBLs are vastly more common than CLLs, and only a tiny fraction of CLLs progress into more advanced stages of the disease or evolve into Richter syndrome or undergo prolymphocytoid transformation. Shaded triangle represents the progressively decreasing size of individual situations. HSCs of CLL patients have been suggested to carry intrinsic abnormalities and to be skewed toward B-cell lineage (top left corner). The encounter of such a B cell with an appropriate external stimulus (here exemplified with the antigen/BCR interaction) triggers the clonal development of MBLs, probably enhancing the cell stimulation. The possibility exists (bottom left corner) that external stimulation may precede the appearance of genetic anomalies. Over time, the pressure of (eg, antigenic) stimulation leading to enhanced proliferation favors the acquisition of (initial or additional) genetic abnormalities, and some MBLs may progress into overt CLL. If and when the acquired genetic abnormalities (eg, recurrent mutations) are able to substitute the necessity of external stimulation by impinging on the same final pathway(s), a more aggressive version of CLL is acquired (either in the form of refractory disease or of Richter syndrome or of prolymphocytoid transformation).
The progressive evolution from antigen (Ag) stimulation to molecular abnormalities in the natural history of CLL. MBLs are vastly more common than CLLs, and only a tiny fraction of CLLs progress into more advanced stages of the disease or evolve into Richter syndrome or undergo prolymphocytoid transformation. Shaded triangle represents the progressively decreasing size of individual situations. HSCs of CLL patients have been suggested to carry intrinsic abnormalities and to be skewed toward B-cell lineage (top left corner). The encounter of such a B cell with an appropriate external stimulus (here exemplified with the antigen/BCR interaction) triggers the clonal development of MBLs, probably enhancing the cell stimulation. The possibility exists (bottom left corner) that external stimulation may precede the appearance of genetic anomalies. Over time, the pressure of (eg, antigenic) stimulation leading to enhanced proliferation favors the acquisition of (initial or additional) genetic abnormalities, and some MBLs may progress into overt CLL. If and when the acquired genetic abnormalities (eg, recurrent mutations) are able to substitute the necessity of external stimulation by impinging on the same final pathway(s), a more aggressive version of CLL is acquired (either in the form of refractory disease or of Richter syndrome or of prolymphocytoid transformation).
A plausible working hypothesis
It is reasonable to postulate that the evolution of MBL follows a pattern reminiscent of human polygenic nonmalignant diseases where tilting the balance toward a more or less aggressive disease depends on which “passenger” or influential genes are turned on and also on which environmental elements are coming into action.59 We have therefore to start asking whether the concept of driver and passenger genes applies to CLL and how it may relate to the disease development.
Genome-wide sequencing34,36,38 and animal models indicate that we probably don't have to concentrate on mutations of individual driver genes but rather on the activation of different critical pathways that may be geared into action because of both stimuli originating from the microenvironment surrounding the leukemic cells and/or a number of different genetic abnormalities. Microenvironment-triggered events, represented by Ag-mediated BCR ligation complemented by the stimulation of coreceptors, including CD4047 and TLRs,60 are more likely to occur initially and may be maintained throughout the whole natural history providing a multiplier effect. One may speculate that the repeated/prolonged activation of target cells by daily apoptosis byproducts and/or bacteria elimination may favor cell-cycle entry and increased proliferation, explaining the unusually dynamic kinetic behavior of CLL cells.61
The initial functional activation of survival/proliferation pathways in target cells may later become subsidized by autonomous genetic abnormalities (eg, a single mutation) affecting the same or parallel critical pathway(s). As an example, MyD88 mutations34 may have the same consequences of an unabated cellular stimulation because of infectious/inflammatory agents that trigger TLR.46 As highlighted by the identification of different recurrent mutations,34,36,38 multiple identical or parallel pathways can be involved. This would explain why to date the task of identifying single recurrent and common mutations in CLL remains unaccomplished. It is nevertheless conceivable that all the stimuli coming from multiple identical or parallel pathways may converge to a final common pathway. An important candidate is NFKB,62,63 considering that its abnormalities may be caused by MyD88 and NOTCH1 mutations as well as by the activation induced by microenvironment stimuli, deriving from the activation of the BCR, TLRs, CD40, and CXCR4.64
The conclusion is that we may have different ways to reach the final result of MBLs progressing into CLL. These possibilities span from critical gene mutations to microenvironmental stimulation (including Ag), the latter being perhaps the most frequent causative event, especially if superimposed onto a CLL-prone genetic background (Figure 1). If we accept this reasoning, attempts to prevent Ag stimulation either by antibiotic treatment or preventive vaccination might be helpful in hindering deleterious cellular activation. Attractive, though still speculative, evidence exists in the literature for a link between respiratory infections and increased risk to develop CLL.65 It remains to be demonstrated whether this association may simply reflect underlying immune disturbances present before CLL diagnosis.
The clinical problem
In clinical terms, our biologic ignorance translates into a simple question. What should we do when we encounter a clinical MBL? And how should we behave when we scout a population screening MBL? The most sensible conclusion is that for clinical MBL we have to consider a follow-up MGUS-style,21 first reassuring the affected persons that their risk of progression toward a full-blown leukemia is in the range of 1% to 2% per year and then suggesting a yearly hematologic consultation with a complete blood cell count and whenever deemed useful abdominal ultrasound/chest x-ray, with no invasive investigations whatsoever.33 For population-screening MBL, based on the most recent evidence,20 the risk of progressing and developing CLL is very low, if any, and likely not different from that of the general population. Therefore, the most reasonable attitude is to continue investigating these subjects to establish the genetic architecture of MBL as this would allow defining which genes are critically involved in evolution, which critical pathways are involved, and how they relate to microenvironmental influences. These investigations will solve the question of whether the investigation of MBL is pursuing a right track or whether at least some MBLs are irrelevant. In any case, defining which elements are meaningful in the transition of MBL into overt CLL and therefore pinpointing which MBL, should alert the physician would represent a major achievement. If the current concept that inflammatory/infectious agents may play a role in the appearance of CLL-like cells will turn to have solid ground, pilot trials investigating either vaccination strategies or antibiotics/anti-inflammatory prophylaxis could be proposed.
Acknowledgments
This work was supported by Associazione Italiana per la Ricerca sul Cancro (Program Molecular Clinical Oncology-5 per mille no. 9965 and Investigator Grant), United States/European Alliance for the Therapy of CLL, CLL Global Research Foundation, Cariplo Foundation, Progetti di Ricerca di Interesse Nazionale, Ministero dell'Istruzione Universitá Ricerca, and Ricerca Finalizzata Ministero della Salute, Rome, Italy.
Authorship
Contribution: P.G. searched the literature, wrote the manuscript, and drew the figure; and F.C.-C. discussed the current literature, wrote the manuscript, and drew the figure.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paolo Ghia, Department of Onco-Hematology, Via Olgettina 60, 20132, Milano, Italy; e-mail: ghia.paolo@hsr.it.