Abstract
Over the past 10 years, significant progress has been made in understanding HIV-associated lymphomas and improving the prognosis of these diseases. With the advent of combination antiretroviral therapy and the development of novel therapeutic strategies, most patients with HIV-associated lymphomas are cured. The outcome for the majority of patients with HIV-associated diffuse large B-cell lymphoma and Burkitt lymphoma in particular, is excellent, with recent studies supporting the role of rituximab in these diseases. Indeed, in the combination antiretroviral therapy era, the curability of many patients with HIV-associated lymphoma is similar to their HIV-negative counterparts. New treatment frontiers need to focus on improving the outcome for patients with advanced immune suppression and for those with adverse tumor biology, such as the activated B-cell type of diffuse large B-cell lymphoma and the virally driven lymphomas. Future clinical trials need to investigate novel targeted agents alone and in combination with chemotherapy.
Introduction
Lymphomas are an important complication of HIV infection where they occur with high frequency and are a significant cause of morbidity and mortality. Most of these are aggressive B-cell lymphomas and are histologically heterogeneous and include lymphomas that are commonly diagnosed in HIV-negative patients as well as others that are primarily associated with HIV infection and occur in patients with severe immunodeficiency. The common HIV-associated lymphomas are diffuse large B-cell lymphoma (DLBCL), which includes primary CNS lymphoma (PCNSL), and Burkitt lymphoma (BL), whereas primary effusion lymphoma (PEL), plasmablastic lymphoma and classic Hodgkin lymphoma (HL) are far less frequent. Other lymphoma subtypes, such as follicular lymphoma and peripheral T-cell lymphoma, can also be seen but are quite uncommon. Epidemiologically, HIV-positive patients have a 60- to 200-fold increased incidence of non-Hodgkin lymphoma (NHL), the majority of which are DLBCL.1 The reader is referred to the current WHO Classification for further details, where HIV-associated lymphomas are separately classified under “Immunodeficiency-associated lymphoproliferative disorders.”2
Since the introduction of combination antiretroviral therapy (CART) in the mid-1990s, HIV-associated lymphomas have fallen in incidence and improved in outcome, in large part because of better control of HIV replication and improved immune function. The risk of systemic or primary CNS lymphoma in HIV-infected persons is closely associated with the CD4 count. In one study, the incidence of systemic lymphoma rose from 15.6 to 253.8 per 10 000 person-years and PCNSL from 2 to 93.9 per 10 000 person-years in patients with a CD4 cell count of more than 350 cells/μL compared with patients with less than 50 cells/μL, respectively.3 In addition, since the widespread use of CART, the proportion of patients in lower CD4 strata has fallen significantly, which has been accompanied by a shift in histologic subtype away from lymphomas, such as PCNSL and PEL, which occur in patients with advanced immunodeficiency, toward BL and HL that occur in patients with higher CD4 counts and better immune function.2,4,5 This pathobiologic shift in tumor types in the era of CART has important implications for outcome as the subtypes of lymphoma that arise in the setting of high CD4 counts are more favorable.
Pathobiology
The pathogenesis of HIV-associated lymphoma involves a complex interplay of biologic factors, such as chronic antigen stimulation, coinfecting oncogenic viruses, genetic abnormalities, and cytokine dysregulation. Most of these lymphomas are of B-cell lineage and harbor clonal rearrangement of immunoglobulin genes. Occasional T-cell lymphomas are observed and would have T-cell receptor gene rearrangements.2,6
Etiology
Chronic antigen stimulation, which is associated with HIV infection, can lead to polyclonal B-cell expansion and probably promotes the emergence of monoclonal B cells.
Recently, circulating free light chains were found to be elevated in patients at increased risk of HIV-associated lymphomas and may represent markers of polyclonal B-cell activation. In the future, they may be useful for the identification of HIV-infected persons at increased risk for the development of lymphoma.7
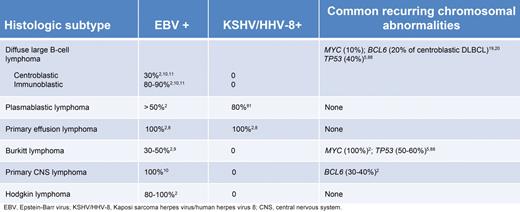
EBV is the most commonly found oncogenic virus in HIV-associated lymphomas and is present in approximately 40% of cases.2 Nearly all cases of PCNSL and HL harbor EBV as do 80% to 90% of DLBCL cases with immunoblastic features. Most cases of PEL also harbor EBV in addition to Human herpesvirus 8 (HHV-8), which is present in virtually all cases.8 In contrast, EBV is variably present in BL (30%-50%) and plasmablastic lymphoma (50%) and usually absent in centroblastic lymphomas.9-11 EBV-positive HIV-associated lymphomas frequently express the EBV-encoding transforming antigen latent membrane protein 1, which activates cellular proliferation through the activation of the NF-κB pathway and may induce BCL2 overexpression, promoting B-cell survival, and lymphomagenesis.12-14
Molecular genetics
There are a number of well-defined genetic abnormalities in HIV-associated lymphoma (Table 1). BL is associated with activation of the MYC gene and, when associated with HIV infection, resembles sporadic rather than endemic BL.15 Interestingly, studies have suggested that up to 20% of HIV-positive DLBCLs also harbor a MYC translocation, and this raises the question of whether some of these may be biologically closer to BLs, as recently described in HIV-negative patients.16,17 BCL6 mutations are found in 20% of centroblastic DLBCL and 60% of PEL lymphoma cases.18-20 Finally, cytokine and chemokine dysregulation, such as IL-6 and IL-10, are associated with EBV and HHV-8–associated lymphomas and probably play an important and permissive role in lymphomagenesis.2
Although in-depth gene-expression profiling of HIV-associated lymphomas has not been performed, their molecular profiles are probably similar to DLBCL and BL in HIV-negative patients.21 Early gene-expression profiling studies in untreated DLBCL identified distinct molecular subtypes with different oncogenic abnormalities.22 Genes associated with germinal center B-cell (GCB) DLBCL included markers of germinal center differentiation, such as CD10 and BCL6, whereas those associated with activated B-cell like (ABC) DLBCL included IRF4/MUM1.23-25 A noteworthy feature of ABC DLBCL was the very high expression of BCL2: ABC DLBCL had more than 4-fold higher expression than GCB DLBCL.22,26 These results suggested that the GCB and ABC DLBCL subtypes are derived from B cells at different stages of differentiation, with GCB DLBCL arising from germinal center B cells and ABC DLBCL from post–germinal center B cells blocked during plasmacytic differentiation.
Genetic analysis has revealed ABC and GCB DLBCL to be pathogenetically distinct. GCB DLBCL is exclusively associated with 2 recurrent oncogenic events: the t(14;18) translocation involving the BCL2 gene and the immunoglobulin heavy chain gene and amplification of the c-rel locus on chromosome 2p. They also have amplification of the oncogenic mir-17-92 microRNA cluster, deletion of the tumor suppressor PTEN, and frequent abnormalities of BCL6.27,28 ABC DLBCLs have frequent amplification of the oncogene SPIB, deletion of the INK4a/ARF tumor suppressor locus and trisomy 3, and constitutive activation of the NF-κB pathway, in most cases.29-31 This has been linked to abnormalities in several upstream proteins, including CARD11, BCL10, and A20, leading to activation of IκB kinase and NF-κB.27,30
BL can be readily distinguished from DLBCL by the high level of expression of MYC target genes, the expression of a subgroup of germinal center B-cell genes, and the low level of expression of MHC class I genes and NF-κB target genes.16,17 A few small studies that have looked at gene expression profiling of PEL found its profile to be similar to the non-GCB type of DLBCL and probably of plasmablastic derivation.32,33
Diagnosis and evaluation
The most important diagnostic test is an adequate and properly evaluated biopsy; in general, excisional biopsies should be performed and core or fine needle aspiration biopsies are for the most part inadequate. The tissue evaluation should be performed by an experienced hematopathologist. Whereas many of these lymphomas will be histologically similar to those that develop in HIV-negative patients, others, such as PEL and plasmablastic lymphoma, are observed mostly in HIV-infected patients.
Histology
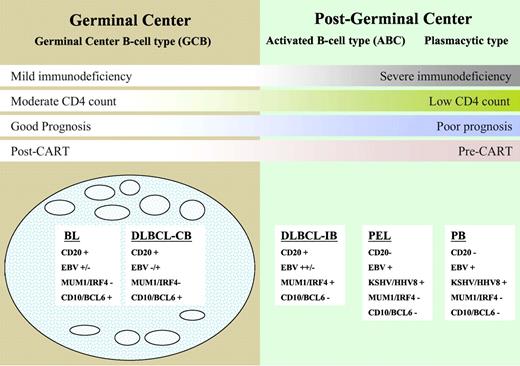
HIV-associated DLBCL is divided into centroblastic and immunoblastic variants. The centroblastic type is characterized by diffuse sheets of large lymphoid cells with round or oval nuclei and prominent nucleoli. They often express germinal center-associated markers, such as CD10 and BCL6, and are typically CD20 positive2,15,34 (Figure 1). The immunoblasic variant refers to those cases containing more than 90% immunoblasts and often exhibits features of plasmacytoid differentiation that may confound the distinction from plasmablastic lymphomas.2,35,36 These tumors are CD10-negative, being of post–germinal center derivation, and frequently positive for MUM1/IRF4 and CD138/syndecan-1, markers associated with plasma cell derivation.15 These tumors have frequent mitoses with high Ki-67/MIB-1 scores.37 The centroblastic type represents approximately 25% of HIV-associated lymphomas, whereas the immunoblastic type represents approximately 10%. In immunoblastic lymphoma, tumor cells may lose CD20 expression because of coexpression of EBV. Markers associated with activation, such as CD30, CD38, and CD71, are often expressed in immunoblastic types.2,5
A model for the histogenesis of HIV-associated lymphomas showing molecular and viral pathogenesis and DLBCL taxonomy. BL indicates Burkitt lymphoma; DLBCL, diffuse large B-cell lymphoma; CB, centroblastic; IB, immunoblastic; PEL, primary effusion lymphoma; and PB, plasmablastic lymphoma.
A model for the histogenesis of HIV-associated lymphomas showing molecular and viral pathogenesis and DLBCL taxonomy. BL indicates Burkitt lymphoma; DLBCL, diffuse large B-cell lymphoma; CB, centroblastic; IB, immunoblastic; PEL, primary effusion lymphoma; and PB, plasmablastic lymphoma.
The neoplastic cells in PEL range in appearance from large immunoblastic to anaplastic large cell lymphoma-type cells. Although this tumor is of B-cell origin, surface B-cell antigens, such as CD20 and CD79a, are not expressed. CD45, CD30, CD38, and CD138 are usually expressed and as discussed earlier are associated with KSHV/HHV-8 and EBV sometimes.33 Finally, plasmablastic lymphomas, which typically occur in the oral cavity or jaw, are usually positive for CD38, CD138, and MUM1/IRF4 and negative for CD20 and CD45.36 EBV is present in greater than 50% of cases, and these tumors appear to have biologic overlap with PEL.33,38
HIV-associated BL is divided into 3 separate entities2 : The classic type accounts for approximately 30% of all HIV-associated lymphomas and morphologically resembles classic BL encountered in HIV-negative patients. BL with plasmacytoid differentiation is characterized by medium-sized cells with abundant cytoplasm and is much more commonly seen in the setting of immune deficiency. Other cases show greater nuclear pleomorphism with fewer but more prominent nuclei; and in the past, these were referred to as “atypical” BL. All types have very high mitotic rates with expression of CD19, CD20, CD79a, and CD10 and are negative for BCL2. EBV positivity ranges from 30% in classic BL to 50% to 70% in BL associated with plasmacytoid differentiaton.6,9,39
Classic HL in the setting of HIV is mostly the mixed cellularity subtype, and EBV is positive in virtually all cases.40 Interestingly, in the CART era, there has been a significant increase in nodular sclerosis HL because of a higher proportion of patients in higher CD4 strata.33,41
Although gene-expression profiling is not routinely used in the diagnosis of HIV-associated lymphoma, the cell of origin of DLBCL (GCB or non-GCB) can be reasonably predicted by the expression of 3 surface proteins (CD10, BCL6, and MUM1) on the tumor tissue using immunohistochemistry as described in the Hans algorithm.42 A recent algorithm from Choi incorporates 2 additional antibodies specific to GCB cells (GCET1 and FOXP1) and may improve the predictive accuracy of immunohistochemistry.43 Although the cell of origin appears to be an independent predictor of prognosis, with the ABC subtype (non-GCB) having a worse outcome, both subtypes are treated similarly at present. However, a recent study suggested that bortezomib may improve the outcome of doxorubicin-based treatment of ABC DLBCL and this is being studied in prospective studies.44,45 One diagnostic challenge is identifying MYC+ DLBCL because, like BL, these tumors have a poor outcome with R-CHOP-based treatment.16,17 Thus, it is prudent to perform cytogenetics or FISH for MYC translocations.
Evaluation
Patients should have a comprehensive medical history with attention paid to signs and symptoms of lymphoma, and a detailed HIV history, including prior opportunistic infections and history of HIV resistance, immune function, HIV viral control, and antiretroviral treatment. The physical examination should include a careful assessment of lymph node regions, the liver, and spleen. Relevant laboratory studies include a complete blood count, chemistry profile with lactate dehydrogenase and uric acid levels, CD4 cell count, and HIV viral load. Hepatitis B and C serologies should be assessed. A bone marrow aspirate and biopsy should be performed at initial diagnosis as involvement by lymphoma is found in up to 20% of cases. Patients with aggressive B-cell lymphomas should have a lumbar puncture for analysis of cerebrospinal fluid by flow cytometry and cytology to check for leptomeningeal lymphoma.46
Imaging studies should include CT scanning of the chest, abdomen and pelvis. Radiographic evaluation of the head should also be performed preferably by MRI. Fluoro-deoxyglucose positron emission tomography (FDG-PET) is useful in HIV-negative aggressive lymphomas, but its role in HIV-associated lymphomas is very poorly studied at this point in time. One of the greatest limitations in using PET is that interpretation can be confounded by inflammation from HIV-associated nodal reactive hyperplasia, lipodystrophy, and infections.47,48 Prior experience evaluating FDG-PET in HIV-associated lymphoma is limited to small retrospective series where most scans were not predictive of remission.
The International Prognostic Index (IPI) is the standard prognostic assessment tool in HIV-negative DLBCL. Its applicability to HIV-associated DLBCL, however, is controversial. Whereas in some studies using CHOP or R-CHOP, the IPI score has divided groups prognostically, this has not been the case with DA-EPOCH and in a recent study of short course-EPOCH-R (infusional etoposide, vincristine, and doxorubicin with prednisone, cyclophosphamide, and rituximab) in newly diagnosed HIV-associated DLBCL, the IPI did not predict progression-free survival (PFS) or overall survival (OS).45,49,50 The prognostic importance of CD4 cell count and immune function in HIV-associated DLBCL, neither of which is part of the IPI, is the most likely confounding variable. Patients with CD4 counts less than 100 cells/μL are at increased risk of serious opportunistic infections and death. Furthermore, as noted earlier, patients with severe immune suppression have a higher incidence of immunoblastic subtypes, most of which are of ABC derivation, and a poor outcome compared with patients with preserved immunity, where the GCB subtype is more common.48 Although a recently reported study from the AIDS Malignancy Consortium (AMC) did not find an association between the cell of origin and outcome in HIV-associated DLBCL, their analysis was retrospective and included patients treated with a variety of different regimens.51-53 Involvement of the CNS, which is increased in HIV-associated aggressive B-cell lymphomas, also confers an adverse prognosis.
Therapeutic controversies
The treatment of HIV-associated lymphoma has evolved over the past 30 years in line with improved control of HIV replication and preservation of immune function (Table 2). Over this period, the therapeutic questions were driven by the need to balance the administration of effective cytotoxic treatment with its effect on immune function and infectious complications: (1) Should lower doses of chemotherapy be used to reduce toxicity and immune suppression? (2) What is the role of rituximab and the optimal regimen? (3) Should CART be suspended during lymphoma therapy?
Dose intensity
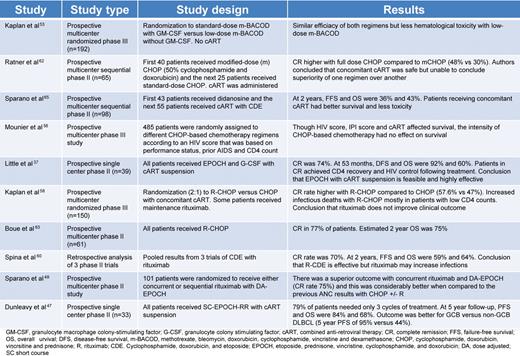
In the pre-CART era, patients with HIV-associated lymphoma had poor outcomes with median survivals of 5 to 6 months. Because these outcomes were driven by both chemotherapy failure and infections, investigators have examined the effect of chemotherapy dose on survival. In one study, Kaplan et al observed that higher doses of cyclophosphamide were associated with lower survival, suggesting that infections were a driving cause of death in these patients.54 In an attempt to reduce infectious deaths, the AMC conducted a study of 192 untreated lymphoma patients randomly assigned to receive standard-dose m-BACOD (methotrexate, bleomycin, doxorubicin, cyclophosphamide, vincristine, and dexamethasone) with GM-CSF support or low-dose mBACOD without GM-CSF in an effort to reduce the toxicity of chemotherapy.55 Compared with full-dose therapy, reduced-dose treatment had a similar response rate (52% vs 41%, respectively) and median survival (6.8 vs 7.7 months, respectively) but lower hematologic toxicity. This led the authors to conclude that lower-dose chemotherapy was preferable in HIV-associated lymphoma. One shortcoming of the study was that, although the authors controlled for the absolute CD4 cell count in the survival analysis, they did not include enough patients with high CD4 counts and, ultimately, could not support a definitive recommendation for this group where the benefit of full-dose chemotherapy on cure of the lymphoma may outweigh the infectious risks.37 In addition, in the post-CART era, the proportion of patients with higher CD4 counts is much larger. Importantly, before completion of this trial, a randomized multicenter study in HIV-negative aggressive lymphoma showed CHOP to be equally effective as mBACOD and less toxic.56 The better therapeutic index of CHOP led to its acceptance as a standard for HIV-associated lymphoma.57
Outcome in the CART era and the role of rituximab
The introduction of CART approximately 15 years ago has had a dramatic effect on the outcome of HIV-associated lymphomas with increases in median survival. Although the reasons are multifactorial, they can be ultimately attributed to salutary effects of CART on immune function. Patients with preserved immune function have a lower risk of infectious complications, thereby enabling optimal chemotherapy administration, and as noted earlier, a more favorable tumor biology.5,37 Interestingly, in one study that looked at risk-adapted intensive chemotherapy in 485 patients with AIDS-related lymphoma, CART was significantly associated with survival, whereas the dose-intensity of CHOP-based therapy was not.58
Although the benefit of rituximab is well established in HIV-negative DLBCL, its role in HIV-associated DLBCL has been controversial.59 This debate stems from an AMC randomized phase 3 study of CHOP with or without rituximab in HIV-associated aggressive lymphomas that found rituximab was associated with significantly more infectious deaths but only a trend in improved tumor control; based on this, the authors concluded that rituximab does not improve the clinical outcome of HIV-associated DLBCL.50 A retrospective analysis of 3 phase 2 trials from Italy, where patients received infusional cyclophosphamide, doxorubicin and etoposide (CDE) with rituximab, also concluded that rituximab might increase infections.60,61 On closer evaluation of the AMC trial, however, the increased infectious deaths occurred primarily in patients with very low CD4 counts, and many patients received “maintenance” rituximab after chemotherapy, which has not been shown to be useful in HIV-negative DLBCL.62 Nonetheless, these shortcomings confound any interpretation that rituximab is not useful in HIV-associated DLBCL.
Subsequent to the AMC study, a French group performed a phase 2 study of CHOP plus rituximab in HIV-associated NHL, and the CR rate of 77% and 2 year-survival rate of 75% suggested that rituximab was beneficial and could be given safely to this group of patients.63 To further address the controversy of rituximab, the AMC performed another randomized phase 2 study. At the time that this study was designed, the results of the EPOCH regimen in this population were very promising, and they randomized patients to receive concurrent versus sequential rituximab with EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and hydroxydaunorubicin).37,51 Importantly, they found that concurrent rituximab was not associated with increased infectious deaths.37,51 The study also examined whether the complete response rate with EPOCH-R was superior to CHOP with or without rituximab, using a predetermined retrospective analysis, and whether concurrent versus sequential rituximab was more toxic and/or more effective. There was no difference in toxicity between the arms and the authors rejected the null hypothesis of 50% (associated with CHOP with or without rituximab) in favor of 75% complete response for EPOCH with concurrent rituximab (P = .005; power 0.89).51 Based on this study, we consider it very unwise to omit rituximab from upfront therapy in HIV-associated lymphoma.
The results of the aforementioned AMC trial and our own findings with EPOCH-based treatment in HIV-associated lymphomas suggest that it may be an optimal treatment regimen.37,47 Although one group demonstrated good efficacy with R-CHOP in a multicenter setting, it is concerning that 15% of enrolled patients were not evaluable for response because of early events or lacking clinical and radiologic evaluations.63 Although the AMC's conclusions regarding EPOCH-R's superiority over R-CHOP are based on a historical comparison, the dramatic differential outcome with these 2 regimens in a similar patient population suggests to us that EPOCH-R is a better regimen in this population. Whether or not there are subgroups of patients with HIV-associated DLBCL who may do as well with R-CHOP is unknown at this time, and we therefore recommend using EPOCH-R in all patients with HIV-associated agressive lymphoma.
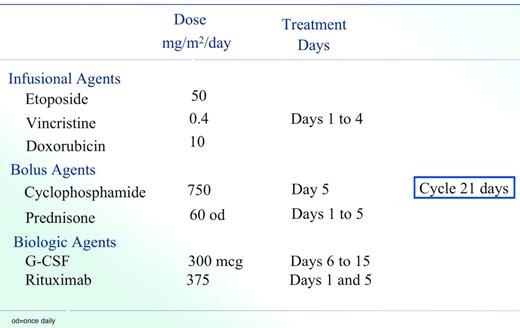
Our current strategy involves a second-generation EPOCH regimen termed Short Course-EPOCH-RR (Figure 2), which is based on the good efficacy and tolerability of DA-EPOCH in this patient population.47 This approach is designed to address the dual challenge of achieving excellent tumor control while preserving immune integrity. Whereas we previously demonstrated that 6 cycles of DA-EPOCH is highly effective (PFS and OS of 73% and 60%, respectively, at 53 months) in HIV-associated lymphoma, we hypothesized that the addition of rituximab would enhance efficacy and allow a significant reduction in treatment cycles.37 With 5 years of follow-up, the PFS and OS of SC-EPOCH-RR are 84% and 68%, respectively, and 79% of patients only required 3 treatment cycles.47 In our study, PFS included patients who relapsed or were refractory or died from lymphoma but HIV-related deaths were censored.
SC-EPOCH-RR drug doses and schedule. SC-EPOCH-RR is administered through a central line. Patients have a complete blood count twice weekly and at least 3 days apart. Cyclophosphamide is reduced 25% for a nadir absolute neutrophil count (ANC) less than 0.5 × 109/L (500/mm3) or platelet count less than 25.0 × 109/L (25 000/mm3) lasting 2 to 4 days and 50% if the nadir ANC was less than 0.5 × 109/L (500/mm3) or platelet count less than 25.0 × 109/L (25 000/mm3) lasting for 5 or more days, based on twice weekly blood counts.
SC-EPOCH-RR drug doses and schedule. SC-EPOCH-RR is administered through a central line. Patients have a complete blood count twice weekly and at least 3 days apart. Cyclophosphamide is reduced 25% for a nadir absolute neutrophil count (ANC) less than 0.5 × 109/L (500/mm3) or platelet count less than 25.0 × 109/L (25 000/mm3) lasting 2 to 4 days and 50% if the nadir ANC was less than 0.5 × 109/L (500/mm3) or platelet count less than 25.0 × 109/L (25 000/mm3) lasting for 5 or more days, based on twice weekly blood counts.
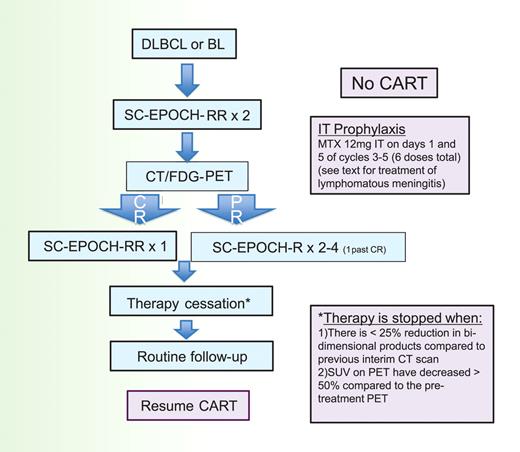
To determine how many cycles of SC-EPOCH-RR are needed, we use the paradigm shown in Figure 3. All patients undergo restaging with CT and FDG-PET scan after the second treatment cycle and each cycle thereafter until achieving a CR or no further tumor shrinkage. The criteria for stopping treatment is when, after a minimum of 3 cycles of therapy, there is less than 25% reduction in bidimensional products compared with the previous interim CT scan and the standardized uptake values on FDG-PET have decreased by at least 50% compared with the pretreatment FDG-PET. All patients receive at least 3 cycles of therapy. Our liberal definition of required standardized uptake value reduction was necessary to take into account HIV-associated reactive changes that confound FDG-PET interpretation in HIV-positive patients.
SC-EPOCH-RR treatment paradigm. Patients receive 2 cycles of SC-EPOCH-RR and are then restaged by CT and FDG-PET scanning. Patients in CR after 2 cycles receive one more cycle (minimum 3) of therapy. Patients with a “positive” CT and/or FDG-PET study after 2 cycles receive additional cycles until they were negative, for a maximum of 6 cycles.
SC-EPOCH-RR treatment paradigm. Patients receive 2 cycles of SC-EPOCH-RR and are then restaged by CT and FDG-PET scanning. Patients in CR after 2 cycles receive one more cycle (minimum 3) of therapy. Patients with a “positive” CT and/or FDG-PET study after 2 cycles receive additional cycles until they were negative, for a maximum of 6 cycles.
We also required that all patients receive intrathecal therapy for prophylaxis of CNS lymphoma; patients receive 12 mg of methotrexate intrathecally on days 1 and 5 of cycle 3, and this is repeated every 3 weeks for a total of 6 doses (ie, cycles 3-5). If patients have active leptomeningeal disease at diagnosis, detected by cytology or flow cytometry, they receive induction intrathecal or intraventricular methotrexate twice weekly for 2 weeks beyond negative flow cytometry (for a minimum of 4 weeks), followed by consolidation weekly for 6 weeks and maintenance monthly for 6 months.46 All patients also receive prophylaxis for Pneumocystis jiroveci and for Mycobacterium avium if the CD4 count at lymphoma diagnosis is less than 100/μL.
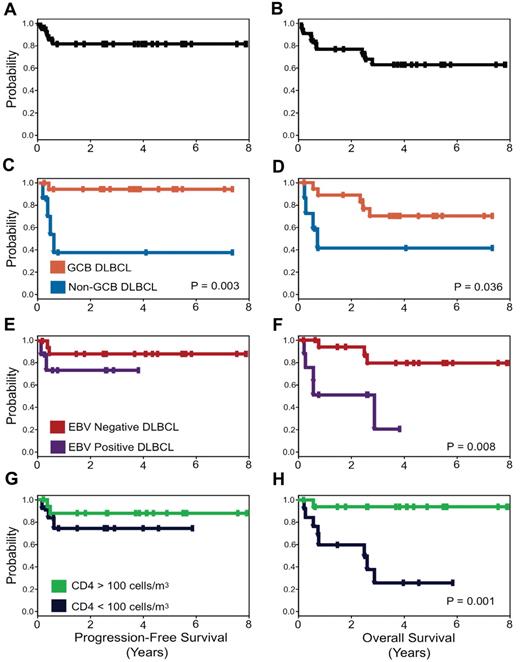
Interestingly, with this approach, the clinical prognostic characteristics that make up the IPI and the IPI itself do not predict PFS or OS. Only tumor histogenesis is associated with lymphoma-specific outcome with 95% of GCB versus 44% of non-GCB DLBCL progression-free at 5 years. Although both EBV positivity of the tumor and low CD4 count at diagnosis are significantly associated with an inferior OS, they are not associated with lymphoma-specific outcome (Figure 4).
PFS and OS Kaplan-Meier curves. PFS (A) is 84% and OS (B) is 68% at the median follow-up of 5 years. PFS (C) and OS (D) for patients with GCB versus non-GCB DLBCL. PFS (E) and OS (F) for EBV-negative versus EBV-positive DLBCL, and PFS (G) and OS (H) for CD4 cell count greater than 100 cells/μL (100 cells/mm3) versus less than 100 cells/μL (100 cells/mm3) at diagnosis.
PFS and OS Kaplan-Meier curves. PFS (A) is 84% and OS (B) is 68% at the median follow-up of 5 years. PFS (C) and OS (D) for patients with GCB versus non-GCB DLBCL. PFS (E) and OS (F) for EBV-negative versus EBV-positive DLBCL, and PFS (G) and OS (H) for CD4 cell count greater than 100 cells/μL (100 cells/mm3) versus less than 100 cells/μL (100 cells/mm3) at diagnosis.
Role of CART during therapy
The risks and benefits of continuing CART during curative chemotherapy of aggressive lymphomas have been variably interpreted. Although many investigators rightly raise the concern that uncontrolled HIV replication during chemotherapy will worsen immune function, they often do not consider the potentially adverse effects of CART on lymphoma-specific outcomes because they are difficult to quantify. One of the first trials to assess concurrent CART was a nonrandomized AMC study of dose reduced and standard dose CHOP.64 A potentially important finding of the study comes from the pharmacokinetic analysis, which showed that cyclophosphamide clearance was reduced 1.5-fold but doxorubicin clearance was unchanged compared with historical results. Although it is reassuring that the doxorubicin pharmacokinetics were unaffected, the reduced clearance of cyclophosphamide, an inactive prodrug, could probably result in a reduction of active metabolites and potentially compromise efficacy. In this study, CD4 counts increased significantly during therapy, and the mechanism for increased CD4 cell counts raises the concern that CART protects T cells from chemotherapy-induced cytotoxic stress, an effect that might occur in the lymphoma cells.65,66 Although other groups have suggested that CART can be safely administered with chemotherapy, it has not been well prospectively studied and controversies abound.67,68 In that respect, it is important to note that many newer antiretrovirals with fewer drug interactions (than those studied in the past) are now available.
Our approach has been to suspend CART during chemotherapy because we think the risk-benefit of CART is not favorable. We are particularly concerned with pharmacokinetic and pharmacodynamic interactions that could lead to lower steady-state drug concentrations, a particular problem with infusional regimens, and/or increase toxicity, which may lead to chemotherapy dose reductions.69,70 Of theoretical but no less important concern is the potential inhibitory effect of some antiretroviral drug classes on lymphoid cell apoptosis and the potential for CART noncompliance, which would increase the risk of developing new HIV mutations.71,72 To assess the risks of CART suspension, we performed 2 prospective studies where CART was suspended during chemotherapy (DA-EPOCH and SC-EPOCH-RR) and did not observe a significant increased risk of infections during therapy.37,47 Although the HIV viral loads rapidly increased and then plateaued after the first cycle and the CD4 cells decreased over the course of chemotherapy, both HIV viral loads and CD4 levels returned to baseline levels a few months after the completion.37,47 Furthermore, there was loss of HIV-viral mutations, which were present before treatment, after completion of EPOCH. Thus, our current approach with SC-EPOCH-RR is to suspend CART from the beginning until the completion of treatment and as 79% of patients require just 3 cycles of therapy, the duration of CART suspension is approximately 7 weeks, in the majority of cases (Figure 3).
HIV-associated BL
Although, after the advent of CART, there was a significant improvement in the outcome of HIV-associated DLBCL, this was not the case initially with HIV-associated BL, as reported in a retrospective series by Lim et al.73 This lack of improvement is probably explained by the widespread use of CHOP-based regimens, which have poor efficacy in BL.16,74 Whereas dose-intense regimens, such as hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) and CODOX-M-IVAC, with or without rituximab, have shown encouraging results in HIV-negative BL, they have not been studied too extensively in HIV-associated BL. One of the concerns with CODOX-M-IVAC is treatment-related toxicity in this population.75,76 In an attempt to reduce this, the AMC recently presented the results of a feasibility and toxicity study for HIV-associated BL and atypical BL and reported good OS rates with 65% of patients completing treatment as per protocol.77
BL highlights the necessity to balance treatment efficacy and toxicity by optimizing the therapeutic index, especially in patients who are immune-suppressed and/or elderly. In this regard, we studied using DA-EPOCH-R in untreated BL based on its excellent activity in highly proliferative DLBCL and its favorable toxicity profile. Among 29 patients (including 10 HIV-positive treated with SC-EPOCH-RR), we observed a complete remission and OS rate of 100% at a median follow-up time of 57 months. The AMC also included several patients with BL or Burkitt-like lymphoma in their study of concurrent versus sequential EPOCH-R and reported high response rates in this group.51 In summary, although modified CODOX-M-IVAC regimens are effective and reasonably well tolerated in this population, our current approach is to use SC-EPOCH-RR for newly diagnosed patients with HIV-associated BL (Figure 2). The treatment paradigm that we use is the same as for DLBCL, with the majority of patients requiring only 3 cycles of therapy and short duration of CART suspension (Figure 3).47 All patients receive prophylactic intrathecal therapy, and those with leptomeningeal disease at diagnosis receive intensive intrathecal therapy for at least 6 months' duration.47 A prospective national study that will test the regimen in both HIV-negative and HIV-positive patients with BL is planned at this time.
Approaches to other HIV-associated lymphomas
HL
In the setting of HIV infection, classic HL occurs most frequently in patients with depressed immune function. However, a paradoxical increase in classic HL has been observed in the CART era despite an overall improvement in immune function in most patients.78 This is probably explained by examining the incidence of the 2 major subtypes of classic HL that occur in HIV infection. In the pre-CART era, most classic HL was mixed cellularity subtype, which is EBV-positive and occurs mostly in immune-suppressed patients, whereas more recently there has been an increased incidence of nodular sclerosis HL, which occurs more commonly at higher CD4 counts.41 When considering treatment, one needs to consider that patients with the mixed cellularity subtype typically have advanced disease, including bone marrow involvement, and require chemotherapy alone. In contrast, patients with nodular sclerosis HL will typically present with mediastinal masses and may benefit from combined modality treatment in selected cases. No studies have adequately evaluated different regimens in HIV-associated HL to make definitive recommendations about regimen efficacy. Thus, we recommend doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) chemotherapy, which is the standard for HIV-negative patients. The impact of CART suspension has not been well studied in HL, but given the relatively long treatment duration and bolus scheduling of ABVD, we recommend that CART be continued.
PCNSL
Primary central nervous system lymphoma (PCNSL) typically presents in patients with severe immune suppression. Thus, it is not unexpected that since the advent of CART, its incidence has decreased dramatically. Although the disease remains incurable in most patients, the duration of survival appears to have increased. Compared with HIV-negative patients, HIV-associated PCNSL is typically EBV-positive.2 Patients frequently present with changes in mental status or focal neurologic symptoms and, unlike HIV-negative PCNSL, they tend to present with multiple brain lesions. Because these patients are severely immune-suppressed, intracranial opportunistic infections should always be considered in the differential diagnosis when evaluating intracranial lesions on imaging studies.
Unlike HIV-negative PCNSL, where high-dose methotrexate and, more recently, combination chemotherapy regimens are effective, total brain irradiation remains standard in HIV-associated PCNSL. Whereas most studies in the pre-CART era report a median survival in the range of 3 months, survival more than 1.5 years has been reported in patients who respond to CART and were treated with radiation.79,80 The role of systemic therapy and rituximab remains undefined in this disease, although some studies are investigating these agents. Our approach is to recommend that these patients be referred for investigational studies or, if unavailable, total brain radiation is reasonable.
PEL and plasmablastic lymphoma
The outcome of PEL is poor with standard treatment and the median survival is in the range of 6 months.81 Unlike some other HIV-associated lymphomas, CART does not appear to have had a significant impact on survival. At this time, the optimal therapy for PEL remains to be defined, but regimens such as EPOCH and CDE may be beneficial. Other approaches, such as high-dose methotrexate and parenteral zidovudine with interferon-α, have been studied but have demonstrated limited efficacy.82,83 The prognosis of plasmablastic lymphoma in the setting of HIV has also been historically poor.38,84 The impact of CART has not been well studied but anecdotal reports suggest its prognosis may have improved since the introduction of CART.85 It is reasonable to consider regimens, such as EPOCH or CDE, for this disease. Newer agents, such as bortezomib and lenalidomide, have been used anecdotally with some reports of activity and success.86
Relapsed lymphoma
Relapsed lymphoma is associated with a poor prognosis, and median survivals tend to be less than 1 year. A recent Italian study prospectively evaluated high-dose therapy and stem cell transplantation in 50 patients with relapsed HIV-associated lymphoma (both HL and NHL).87 Whereas the median OS of patients was 33 months, patients who had chemo-sensitive disease had a relatively favorable outcome and were disease free at 44 months of follow-up. Given the significant improvements in HIV control and immune function, it is reasonable to approach relapsed HIV- associated lymphomas similarly to their HIV-negative counterparts and to pursue aggressive strategies if appropriate. Less aggressive strategies, such as etoposide, solumedrol, high-dose cytarabine, and platinum and CDE, have poor outcomes.61,88 The role of allogeneic transplantation has not been well evaluated at this time.
Future directions
Our approach to treating HIV-associated DLBCL and BL is to use EPOCH-R (with antiretroviral therapy suspension), and preliminary evidence from our institution suggests that abbreviated cycles may be given to further reduce toxicity. For HL, we use ABVD with antiretroviral continuation because of the long duration of therapy. For PCNSL and less common HIV-associated lymphomas, survival with standard approaches to date has been poor and experimental therapy should be considered.
The outcome of HIV-associated lymphoma has undergone significant improvement in recent years beginning with the widespread use of CART. Both DLBCL and BL are highly curable diseases for the most part. To further improve the outcome of these lymphomas, the challenge is to identify driver pathways and therapeutic targets. In this regard, we are investigating modulation of the B-cell receptor cascade and NFκB transcription factor, which are involved in the pathobiology of ABC DLBCL.44,89 For GCB DLBCL and BL, current approaches have excellent efficacy with little room for improvement so that future studies should focus on further reducing treatment toxicity, particularly in highly immune-suppressed patients. Advances in the therapeutics of poor prognostic diseases, such as HIV-associated PCNSL and PEL, which are now much more rarely encountered, will probably come from improved understanding of their pathobiology.
Acknowledgments
This work was supported by the Intramural Research Program of the National Cancer Institute of the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: K.D. and W.H.W. wrote and gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kieron Dunleavy, Metabolism Branch, National Cancer Institute, Bldg 10, Rm 4N-115, 9000 Rockville Pike, Bethesda, MD 20892; e-mail: dunleavk@mail.nih.gov.