Abstract
Donor lymphocyte infusion (DLI), a standard relapse treatment after allogeneic stem cell transplantation (AlloSCT), has limited efficacy and often triggers GVHD. We hypothesized that after AlloSCT tumor-infiltrating donor lymphocytes could be costimulated ex vivo to preferentially activate/expand antitumor effectors. We tested the feasibility and safety of costimulated, tumor-derived donor lymphocyte (TDL) infusion in a phase 1 trial. Tumor was resected from 8 patients with B-cell malignancy progression post-AlloSCT; tumor cell suspensions were costimulated with anti-CD3/anti-CD28 Ab-coated magnetic beads and cultured to generate TDL products for each patient. Costimulation yielded increased proportions of T-bet+FoxP3− type 1 effector donor T cells. A median of 2.04 × 107 TDL/kg was infused; TDLs were well tolerated, notably without GVHD. Two transient positron emission tomography (PET) responses and 2 mixed responses were observed in these refractory tumors. TDL are a feasible, tolerable, and novel donor cell therapy alternative for relapse after AlloSCT. This trial is registered at clinicaltrials.gov as no. NCT00445666.
Introduction
Donor lymphocyte infusion (DLI) is standard relapse treatment after allogeneic hematopoietic stem cell transplantation (AlloSCT), with durable responses reported in relapse of chronic hematologic malignancies, but is not available for cord blood or many unrelated donor recipients. DLI response rates vary but, except for early relapse of chronic myelogenous leukemia, are generally < 50%.1-3 Responses are strongly associated with GVHD, affecting up to 60%, with up to 11% treatment-related mortality.4 We hypothesized that relapsed tumor post-AlloSCT would be infiltrated with tumor-specific donor lymphocytes that might be primed but inadequately costimulated for tumor cell killing. Their costimulation and expansion ex vivo might yield a donor lymphocyte product with enhanced tumor specificity and graft-versus-tumor (GVT) potency. Safety of ex vivo CD3/CD28-costimulated DLI has been demonstrated,5-8 as has feasibility of CD3/CD28-expanded marrow lymphocytes from myeloma patients.9 Preclinical feasibility studies with tumor-infiltrating donor lymphocytes10 provided the basis for a phase 1 clinical trial of tumor-derived donor lymphocyte (TDL; BB-IND13226, MRB) infusion for relapsed B-lymphoid malignancy.
Methods
Eligible adults had: progression of B-cell malignancies despite full-donor T-cell engraftment after AlloSCT; trial of immune suppression withdrawal; DLI (if available); minimal active GVHD; and resectable tumor. The National Cancer Institute Institutional Review Board approved this study, and all subjects provided informed, written consent in accordance with the Declaration of Helsinki. Primary endpoints were feasibility (at least 80% success in generating the TDL product) and safety (≤ 60% grade 2-4 acute/late-acute GVHD within 28 days). Tumor response was a secondary end point. Accrual stopped subsequent to a planned interim analysis of the first 8 of 15 subjects, when the investigator-held investigational new drug application changed institutions.
After surgical tumor harvest, single-cell suspensions were prepared and costimulated by incubating with anti-CD3/anti-CD28 Ab-coated magnetic beads (BB-IND6675, CHJ) at a 3:1 bead/T-cell ratio and expanded in TDL media (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Cultures were harvested when yields reached infusion targets, with ≥ 50% CD3+ T cells and ≤ 5% CD19+ tumor cells by flow cytometry. Expanded TDL products were cryopreserved and tested for release criteria (sterility, donor chimerism > 90%, and purity: residual tumor < 5% by clinical pathology and microscopic bead count < 100 per 3 × 106 cells). TDL dose was determined by culture yield, with 1-100 × 106 TDL/kg infused without additional immunoprophylaxis or exogenous cytokines. Subjects were monitored for toxicity11 for 24 hours in-hospital and at 1, 2, 4, 8, and 12 weeks; response was assessed at 4, 8, and 12 weeks (supplemental Figure 1).12,13
Fresh tumor-infiltrating lymphocytes and the expanded TDL products were phenotyped with multicolor flow cytometry for T-cell and effector subsets; TDL clonality was assessed with PCR for TCR-γ-chain (TRG locus) gene rearrangements and Vβ spectratyping; and tumor-associated Ag–specific CD8+ donor T cells were sought with an MHC/peptide tetramer library.14 Proliferative alloresponses and antitumor cytotoxicity were assessed in mixed lymphocyte culture, with CFSE and caspase-8 cleavage, respectively (supplemental Methods).
Results and discussion
Primary feasibility and safety aims were met with the initial 8 patients (Table 1). All surgically harvested tumors demonstrated donor lymphocyte infiltration; T cells comprised 0.5% to 71% of tumor cell suspensions, with donor origin confirmed by short tandem repeat chimerism analysis. CD3/CD28 costimulation and culture yielded clinical TDL products for 8 of 8 patients (95% CI for feasibility: 63.1%-100%), generating a median of 2.04 × 109 cells in a median of 12 days. A median 95% of TDL were CD3+ with variable expansion of both CD4+ and CD8+ T cells yielding a median CD4/CD8 ratio of 3.7.
T cells with a type 1 effector phenotype predominated in all TDL products (supplemental Figure 2), with the majority expressing the type 1 transcription factor T-bet (86% of CD4+ and 90% of CD8+ T cells), while the proportion of CD4+CD25+FoxP3+ Tregs decreased in all cultures (from median 19% to 3%). Likewise, the proportion of T cells expressing cell-surface markers of effector function increased with costimulation: CD4+ T cells expressing CD40L uniformly increased (from median 1% to 19%); CD8+ T-cell expression of NKG2D generally increased as well, although expression was more heterogeneous at baseline and increased less consistently from tumors with a high initial proportion of CD8+NKG2D+ T cells (from median 13% to 61%). CD27 expression, lost with terminal differentiation of effector T cells, is a proposed marker of T-cell product potential for in vivo persistence and expansion.15,16 Predictably, the frequency of CD27+ T cells decreased with costimulation/expansion, yet a significant proportion of TDL retained CD27 (median 36% of CD4+ and 39% of CD8+ T cells).
As expected, CD3/CD28 costimulation yielded polyclonal TDL products, without evidence of a dominant TRG gene rearrangement or skewing of TCR repertoire by Vβ spectratyping. PN6 and PN8 lymphocytes could be evaluated with HLA-A2/TAA-peptide tetramers for Ag-specific CD8+ T cells; in both, modest TAA-tetramer+ populations could be found in TDL and in recipient peripheral blood. Although no GVHD was observed, alloreactivity could be detected in TDL products with in vitro proliferative responses against third-party targets. Furthermore, coincubation of PN7's TDL with recipient chronic lymphocytic leukemia (CLL) targets also demonstrated a proliferative response, as well as a cytotoxic effect (TDL vs costimulated donor PBL, 28.7% vs 14.8%, respectively), consistent with a small CLL-reactive population and with the minimal clinical response observed in this patient (supplemental Figure 3).
All patients received TDL infusion. Acute toxicity was minimal, with grade 2 cytokine release syndrome in one patient (PN8). None developed GVHD (95% CI for safety: 0%-36.9%). One patient (PN5) developed grade 4 pneumonitis 22 days after infusion, with acute onset of fever, hypoxia, and pulmonary infiltrates surrounding parenchymal tumor (95% CI for incidence of severe adverse events: 0.3%-52.7%). Extensive evaluation was nondiagnostic and rapid resolution followed steroid treatment (Table 1).
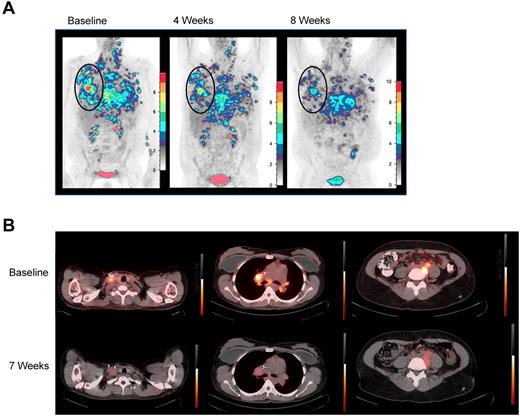
Five patients were evaluable for response. PN1, PN2, and PN3 experienced unabated, rapid progression, and clinical deterioration requiring additional treatment and/or initiation of hospice before 4-week restaging. Four of the 5 subsequent patients maintained stable disease through at least 2 evaluations and each manifested some evidence of antitumor activity. PN6 and PN8 demonstrated transient responses by positron emission tomography (PET), with decreased 18FDG uptake in all measurable lesions (Figure 1), and PN4 and PN7 demonstrated mixed responses. PN5 may also have had an antitumor effect from TDL, if pneumonitis reflected a tumor-directed immune response. Interestingly, both PET responses were in patients with Hodgkin lymphoma (HL) whose tumors were enriched with donor-derived reactive cells. Plausibly, in HL, gain of type 1 effector function and retained tumor homing permitted infused TDL to transiently disrupt the HL microenvironment.17,18
18FDG-PET responses. (A) 18FDG-PET before and at 4 and 8 weeks after TDL. PN6, a 36-year-old with primary-refractory HL with unabated disease progression following myeloablative conditioning and a 6 of 6 HLA-matched sibling-donor AlloSCT with posttransplantation in vivo T-cell depletion with cyclophosphamide. Transient responses followed radiation and gemcitabine, vinorelbine, and liposomal doxorubicin therapies. Twenty-eight months post-AlloSCT, TDL were generated from a right axillary tumor harvest and 26.3 × 106 TDL/kg were infused. Moderate pain and tenderness at sites of chest wall tumor and worsening of pleural effusions developed 1 week after infusion. At restaging, 18FDG-PET-CT demonstrated decreasing standardize uptake values (SUV) in all lesions over 3 consecutive months. By 4 months, however, PET showed increasing SUV in prior sites of disease, which correlated with clinical progression. Shown are coronal maximum intensity projection (MIP) images with a cutoff intensity of 2.5 SUV. (B) Transverse 18FDG-PET-CT fusion images demonstrate decreased SUV in PN8, a 35-year-old with relapsed HL, whose disease progressed from best partial response 6 months after reduced-intensity conditioning and a T-cell replete, 6 of 6 HLA-matched sibling-donor AlloSCT. The patient was treated with vinblastine and subsequently with 3 cycles of dose-adjusted EPOCH (etoposide, vincristine, adriamycin, prednisone, and cyclophosphamide) plus DLI. Seventy months from AlloSCT, TDL were generated from a right axillary tumor harvest, and 91.2 × 106 TDL/kg were infused. Grade 2 cytokine release syndrome (culture-negative fevers to 39.5°C, mild hypotension, and tachycardia) was observed from 4 to 24 hours after infusion. 18FDG-PET-CT was stable at 4 weeks, then demonstrated decrease in SUV in multiple sites of disease at 2 months; tumor size was stable by CT. At 4 months, although PET-CT did not show clear progression, the patient developed severe radicular pain associated with tumor-involved cervical lymph nodes requiring additional therapy.
18FDG-PET responses. (A) 18FDG-PET before and at 4 and 8 weeks after TDL. PN6, a 36-year-old with primary-refractory HL with unabated disease progression following myeloablative conditioning and a 6 of 6 HLA-matched sibling-donor AlloSCT with posttransplantation in vivo T-cell depletion with cyclophosphamide. Transient responses followed radiation and gemcitabine, vinorelbine, and liposomal doxorubicin therapies. Twenty-eight months post-AlloSCT, TDL were generated from a right axillary tumor harvest and 26.3 × 106 TDL/kg were infused. Moderate pain and tenderness at sites of chest wall tumor and worsening of pleural effusions developed 1 week after infusion. At restaging, 18FDG-PET-CT demonstrated decreasing standardize uptake values (SUV) in all lesions over 3 consecutive months. By 4 months, however, PET showed increasing SUV in prior sites of disease, which correlated with clinical progression. Shown are coronal maximum intensity projection (MIP) images with a cutoff intensity of 2.5 SUV. (B) Transverse 18FDG-PET-CT fusion images demonstrate decreased SUV in PN8, a 35-year-old with relapsed HL, whose disease progressed from best partial response 6 months after reduced-intensity conditioning and a T-cell replete, 6 of 6 HLA-matched sibling-donor AlloSCT. The patient was treated with vinblastine and subsequently with 3 cycles of dose-adjusted EPOCH (etoposide, vincristine, adriamycin, prednisone, and cyclophosphamide) plus DLI. Seventy months from AlloSCT, TDL were generated from a right axillary tumor harvest, and 91.2 × 106 TDL/kg were infused. Grade 2 cytokine release syndrome (culture-negative fevers to 39.5°C, mild hypotension, and tachycardia) was observed from 4 to 24 hours after infusion. 18FDG-PET-CT was stable at 4 weeks, then demonstrated decrease in SUV in multiple sites of disease at 2 months; tumor size was stable by CT. At 4 months, although PET-CT did not show clear progression, the patient developed severe radicular pain associated with tumor-involved cervical lymph nodes requiring additional therapy.
Ex vivo expansion of TDL by CD3/CD28 costimulation is feasible, generating large numbers of type 1 effector donor T cells. TDL appear relatively safe; remarkably, no GVHD was observed after infusing these type 1 polarized TDL. Although significant GVHD did exclude enrollment, 4 of 8 subjects had received in vivo T cell–depleted allografts so likely were at significant risk for DLI-associated GVHD. TDL may be a therapeutic option for cord blood recipients and others without access to DLI. Suggestive tumor-directed biologic effects and potential GVT activity in these few, heavily treated patients warrants further investigation of this therapeutic approach. Planned evaluation of pre-TDL immune depletion to support TDL expansion and effector function in vivo may improve magnitude and durability of responses.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the critical contributions of the following current and former coworkers: Charles “Charley” Carter (deceased), E. J. Read, Mark Dudley, and Steven A. Rosenberg provided assistance in development of cell processing methods; Richard Sherry performed surgical tumor harvests; Ellen Mann coordinated tumor specimen acquisition; Mark Raffeld performed PCR on TDL for TCR-γ-chain gene rearrangements, and Armando Filie provided cytopathologic assessment of TDL products from HL tumors. They thank innumerable clinical staff of the National Institutes of Health (NIH) Clinical Center for their outstanding patient care. Finally, they express sincere gratitude to the patients and their families for their generous, essential, and invaluable contributions to this research.
This work was supported by the Intramural National Cancer Institute, Center for Cancer Research (Bethesda, MD).
National Institutes of Health
Authorship
Contribution: N.M.H., S.M.S., R.E.G., D.H.F., and M.R.B. contributed to study conception and design; N.M.H., S.P., B.B.-S., D.N.A., R.J.K., H.M.K., M.S.-S., E.M., A.J.D., B.L.L., C.H.J., F.T.H., and M.R.B. provided study materials and/or patients; N.M.H., V.F., J.J.R., J.O., S.P., S.M., R.R., R.H.V., F.T.H., and M.R.B. collected and assembled data; N.M.H., V.F., J.J.R., S.M.S., S.M., R.R., R.H.V., D.H.F., F.T.H., and M.R.B. analyzed and interpreted data; N.M.H. wrote the manuscript; and all authors reviewed the final manuscript.
Conflict-of-interest disclosure: C.H.J. has received royalties from United States government–owned patents and patent applications in the field of adoptive immunotherapy; this arrangement is under compliance with the policies of the University of Pennsylvania. The remaining authors declare no competing financial interests.
Correspondence: Nancy M. Hardy, MD, Experimental Transplantation and Immunology Branch, CCR, NCI, 10 Center Dr, Hatfield Clinical Research Center, Room 3E-3330, Bethesda, MD 20892-1203; e-mail: hardyn@mail.nih.gov.