To the editor:
Endothelial cell protein C receptor (EPCR) is the cellular receptor for protein C (PC) and activated protein C (APC). Studies from our laboratory1 and others2,3 have shown that FVII/FVIIa also binds to EPCR in a true-ligand fashion. Recently, it has been suggested that FX4 and FXa5 can bind to EPCR and these interactions may play a role in regulating TF-FVIIa-FXa– or FXa-mediated cell signaling via activation of protease activated receptors (PARs). This reported observation that FX/FXa binds to EPCR is somewhat surprising given that the specific Gla domain residues of protein C necessary for molecular recognition of EPCR are not completely conserved in human FX.2 As reported recently,5 if FXa binds to EPCR with an affinity similar to that of APC binding to EPCR, then this interaction may not only influence the activation of FX and FXa-mediated cell signaling, but could also modulate EPCR-dependent functions of APC and FVIIa by competing with APC and FVIIa for EPCR binding.
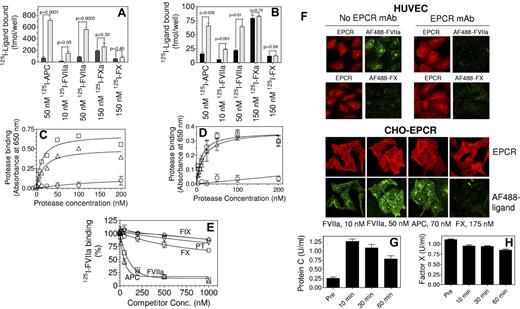
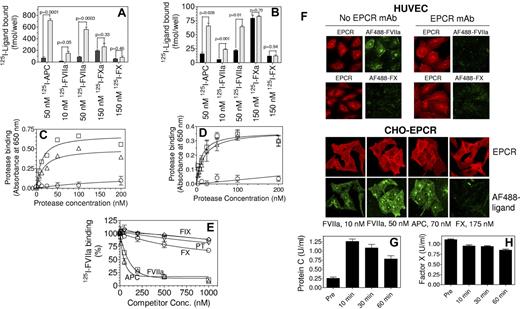
To evaluate the physiologic relevance of FX binding to EPCR, we have reexamined FX binding to EPCR on endothelial as well as CHO-EPCR cells using plasma concentrations of FX and multiple experimental approaches. Radioligand binding studies did not indicate any significant difference in the binding of FX or FXa between CHO and CHO-EPCR cells (Figure 1A). Furthermore, pretreatment of CHO or CHO-EPCR cells with an EPCR blocking mAb did not diminish the basal binding of FX or FXa to these cells (data not shown). Similarly, blockade of EPCR on HUVECs with this EPCR blocking mAb did not have a significant effect on FX or FXa binding (Figure 1B). FVIIa and APC binding studies performed in parallel with FX or FXa experiments clearly demonstrated that both FVIIa and APC bound to cells to a similar degree and in an EPCR-specific manner (Figure 1A-B). Analysis of the binding of biotinylated, active-site blocked FXa, FVIIa and APC to EPCR on CHO-EPCR and HUVECs revealed that little FXa was bound to EPCR on cell surfaces compared with FVIIa or APC (Figure 1C-D). In this assay, FVIIa and APC both bound to EPCR with an apparent Kd of ∼ 15 to 25nM, whereas the Kd for FXa was > 1μM. Consistent with these data that FX does not bind appreciably to EPCR, even a 100-fold molar excess of unlabeled FX (1μM) failed to compete effectively with the binding of 125I-FVIIa (10nM) to CHO-EPCR cells (Figure 1E). Analysis of FX binding to EPCR expressing cells by confocal fluorescence microscopy did not show any detectable fluorescence, either at the cell surface or intracellularly, in CHO-EPCR cells or HUVECs exposed to FX tagged with a fluorescence dye (AF488; Figure 1F). In additional studies, we measured plasma levels of mouse factor X and protein C in EPCR overexpressing mice that received a high dose of active-site inhibited human APC. EPCR overexpression has been found to decrease circulating levels of protein C, while administration of human protein C has been shown to increase mouse protein C levels in circulation by displacing endogenous protein C from EPCR on the endothelium.6 As expected from this study,6 administration of human APCi increased plasma levels of mouse protein C (Figure 1G). In contrast to protein C, there was not a detectable increase in plasma levels of mouse FX after APCi administration (Figure 1H). These data indicate that FX does not effectively interact with EPCR in vivo, at least in regards to the mouse system. Overall our data indicate that FX binding to EPCR, if any, is minimal and likely physiologically insignificant. Our findings do not exclude the possibility that FX/FXa could indirectly interact with EPCR as suggested by others.7
Binding of factors X, Xa, VIIa, and APC to EPCR expressing cells. (A) CHO cells (black bars) or CHO cells stably transfected to express human EPCR (CHO-EPCR; gray bars) were incubated with 125I-labeled human FVIIa (10nM and 50nM), human APC (50nM), human FXa (150nM), or human FX (150nM) in HEPES buffer (10mM HEPES, 0.15M NaCl, 4mM KCl, and 11mM glucose) containing CaCl2 (5mM), MgCl2 (1mM), and BSA (1 mg/mL) for 3 hours at 4°C. At the end of incubation, cells were washed 4 times with the same buffer and surface bound ligands were eluted with glycine (100mM, pH 2.3) and counted for radioactivity to determine the amount of ligand bound to the cells. (B) Same as panel A except that CHO and CHO-EPCR cells were replaced with HUVECs pretreated with EPCR blocking mAb (black bars) or control vehicle (gray bars), respectively. (C) CHO or CHO-EPCR cells were incubated with various concentrations of biotinylated active site-blocked FVIIa (□), APC (Δ) or FXa (○) in Ca2+/Mg2+ containing buffer for 3 hours at 4°C. After washing the cells to remove unbound ligands, the surface bound ligands were detected by fixing the cells and adding alkaline phosphatase coupled streptavidin followed by BluePhos phosphatase substrate system (KPL). EPCR-specific binding was calculated by subtracting the absorbance measured in CHO cells from that of CHO-EPCR cells. Biotinylated active site-inhibited FXa, FVIIa, or APC were prepared by incubating FXa, FVIIa, or APC with 10-fold molar excess of biotinylated EGR for 3 hours at room temperature and excess probe was removed by dialysis. (D) Same as panel C except that CHO and CHO-EPCR cells were substituted with HUVECs pretreated with EPCR blocking mAb or a control vehicle, respectively, and EPCR-specific binding was calculated by subtracting the absorbance obtained with HUVECs pretreated with EPCR blocking antibody from HUVECs not treated with the antibody. (E) CHO-EPCR cells were incubated with 125I-FVIIa (10nM) in the presence of various concentrations of unlabeled competitors, FIX (◇), prothrombin (PT, ▿), FX (○), FVIIa (□), APC (Δ) for 3 hours at 4°C. The surface bound 125I-FVIIa was eluted with low pH glycine and counted for radioactivity. (F) HUVECs, treated EPCR blocking mAb (25 μg/mL) or control vehicle (top panel) or CHO-EPCR cells (bottom panel) were exposed to FVIIa (50nM for HUVECs; 10 and 50nM for CHO-EPCR cells), APC (70nM) or FX (175nM) conjugated with fluorescent probe AF488 for 1 hour at room temperature. At the end of 1 hour, cells were washed quickly and immunostained with nonblocking EPCR mAb. Images through z-axis were acquired using LSM 510 Meta confocal system (Carl Zeiss) and reconstructed to 3D composite images. (G,H) EPCR overexpressing mice were injected intravenously with active site-inhibited human APCi (400 μg/mice) and blood samples were drawn retroorbitally pre- and postadministration of APCi. Mouse protein C level in plasma was measured using mouse protein C–specific ELISA (G) and mouse FX level was measured in a FX specific clotting assay (H).
Binding of factors X, Xa, VIIa, and APC to EPCR expressing cells. (A) CHO cells (black bars) or CHO cells stably transfected to express human EPCR (CHO-EPCR; gray bars) were incubated with 125I-labeled human FVIIa (10nM and 50nM), human APC (50nM), human FXa (150nM), or human FX (150nM) in HEPES buffer (10mM HEPES, 0.15M NaCl, 4mM KCl, and 11mM glucose) containing CaCl2 (5mM), MgCl2 (1mM), and BSA (1 mg/mL) for 3 hours at 4°C. At the end of incubation, cells were washed 4 times with the same buffer and surface bound ligands were eluted with glycine (100mM, pH 2.3) and counted for radioactivity to determine the amount of ligand bound to the cells. (B) Same as panel A except that CHO and CHO-EPCR cells were replaced with HUVECs pretreated with EPCR blocking mAb (black bars) or control vehicle (gray bars), respectively. (C) CHO or CHO-EPCR cells were incubated with various concentrations of biotinylated active site-blocked FVIIa (□), APC (Δ) or FXa (○) in Ca2+/Mg2+ containing buffer for 3 hours at 4°C. After washing the cells to remove unbound ligands, the surface bound ligands were detected by fixing the cells and adding alkaline phosphatase coupled streptavidin followed by BluePhos phosphatase substrate system (KPL). EPCR-specific binding was calculated by subtracting the absorbance measured in CHO cells from that of CHO-EPCR cells. Biotinylated active site-inhibited FXa, FVIIa, or APC were prepared by incubating FXa, FVIIa, or APC with 10-fold molar excess of biotinylated EGR for 3 hours at room temperature and excess probe was removed by dialysis. (D) Same as panel C except that CHO and CHO-EPCR cells were substituted with HUVECs pretreated with EPCR blocking mAb or a control vehicle, respectively, and EPCR-specific binding was calculated by subtracting the absorbance obtained with HUVECs pretreated with EPCR blocking antibody from HUVECs not treated with the antibody. (E) CHO-EPCR cells were incubated with 125I-FVIIa (10nM) in the presence of various concentrations of unlabeled competitors, FIX (◇), prothrombin (PT, ▿), FX (○), FVIIa (□), APC (Δ) for 3 hours at 4°C. The surface bound 125I-FVIIa was eluted with low pH glycine and counted for radioactivity. (F) HUVECs, treated EPCR blocking mAb (25 μg/mL) or control vehicle (top panel) or CHO-EPCR cells (bottom panel) were exposed to FVIIa (50nM for HUVECs; 10 and 50nM for CHO-EPCR cells), APC (70nM) or FX (175nM) conjugated with fluorescent probe AF488 for 1 hour at room temperature. At the end of 1 hour, cells were washed quickly and immunostained with nonblocking EPCR mAb. Images through z-axis were acquired using LSM 510 Meta confocal system (Carl Zeiss) and reconstructed to 3D composite images. (G,H) EPCR overexpressing mice were injected intravenously with active site-inhibited human APCi (400 μg/mice) and blood samples were drawn retroorbitally pre- and postadministration of APCi. Mouse protein C level in plasma was measured using mouse protein C–specific ELISA (G) and mouse FX level was measured in a FX specific clotting assay (H).
Authorship
Contribution. P.S. conducted the binding studies and analyzed the data; R.N. performed confocal microscopy; C.C. performed some of the binding studies; RG conducted animal experiments; C.T.E. provided EPCR overexpressing mice and critical reagents; U.R.P. contributed to the experimental design; and L.V.M.R. designed the research and wrote the manuscript. All authors contributed in writing the manuscript.
Conflict-of-interest disclosure. The authors declare no competing financial interests.
Correspondence: L. Vijaya Mohan Rao, UT Health Science Center at Tyler, 11937 US Highway 271, Tyler, TX, 75708; e-mail: vijay.rao@uthct.edu.