To the editor:
The most common mutation in myeloproliferative neoplasm (MPN) substitutes a valine to phenylalanine at position 617 of JAK2 (JAK2V617F).1 Recently, Rinaldi et al reported both a nuclear and cytoplasmic localization of JAK2 in K562 cells.2 A previous report also indicated that JAK2V617F translocates to the nucleus, where it is proposed to phosphorylate histone H3.3 These findings remain controversial, in part because of nonspecific binding of the commercial anti-JAK2 antibodies used in most studies.4-6 To avoid reliance on antibodies, we also studied the cellular localization of JAK2 wild-type (WT) and V617F expressed as chimeric proteins fused to visible fluorescent proteins (VFP; derivatives of green fluorescent protein). Transfections were performed using an Amaxa electroporation method (Lonza AG) with at least a 60% rate of transfection efficiency in each cell line.
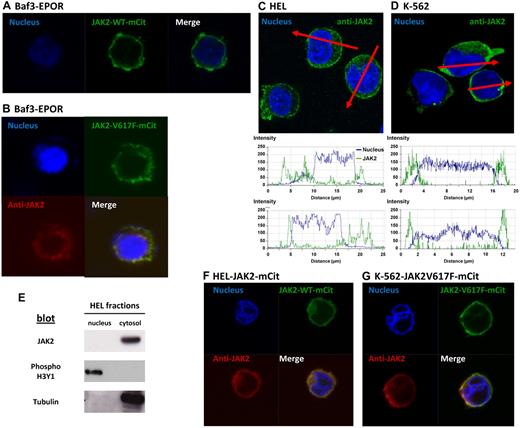
Confocal images of Baf3/EpoR transiently transfected with JAK2WT-VFP or JAK2V617F-VFP constructs showed both fluorescent proteins localized predominantly on the plasma membrane, with a faint signal in the cytosol and no fluorescent signal in the nucleus (Figure 1A-B). Addition of erythropoietin did not induce JAK2 nuclear translocation (not shown). The nuclear dye Hoechst was used as a positive control to stain nuclei in these experiments. Similar results were obtained with different plasmids (pcDNA3.1 or pEf), regardless of the VFP derivative used (mCitrine or YFP). Z-stack analysis ruled out true nuclear localization of occasional spots over the nucleus. We also detected no significant staining for JAK2 in the nuclei of Baf3 cells labeled with primary antibodies specific for JAK2 (Cell Signaling Technology, #3230), and secondary anti–rabbit Cy5-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories). These antibodies were also used in previous studies.3
JAK2 targets the membrane of Baf3 and HEL cells and is excluded from the nucleus. (A) Confocal fluorescent images in Baf3/EPOR cells after transient transfection with JAK2WT-mCitrine. The nucleus is labeled with Hoechst. (B) Confocal images of Baf3/EpoR cells transiently transfected with JAK2V617F-mCitrine (green fluorescence). Cells were also stained with anti-JAK2 (Cy5, red fluorescence) and Hoechst. (C-D) Analysis of endogenous JAK2 distribution in HEL and K562 cells labeled with Hoechst and anti-JAK2 antibodies. Red lines indicate lines drawn across individual cells in the confocal image, with matching plots below reporting fluorescence intensity. (E) Analysis of nuclear and cytosol extracts from HEL cells, subjected to SDS-PAGE, Western blotting and immunoblotting with JAK2, tubulin, and phospho-histone 3 antibodies.(F-G) Confocal fluorescent images in HEL and K562 cells after transient (HEL) or stable (K562) transfection with JAK2V617F-mCitrine (HEL) or JAK2WT-mCitrine (K562) (green fluorescence). Nuclei were stained with Hoechst and endogenous JAK2 was stained with an anti-JAK2 antibody detected by a secondary antibody coupled to a Cy5 dye. Confocal fluorescent images were acquired using a Zeiss LSM 510 META confocal microscope with a 63× oil objective. ZEN 2009 5.5 SP1 software was used for both image acquisition and processing. Before imaging, treated cells were mounted on slides using ProLong Gold antifade reagent (Invitrogen).
JAK2 targets the membrane of Baf3 and HEL cells and is excluded from the nucleus. (A) Confocal fluorescent images in Baf3/EPOR cells after transient transfection with JAK2WT-mCitrine. The nucleus is labeled with Hoechst. (B) Confocal images of Baf3/EpoR cells transiently transfected with JAK2V617F-mCitrine (green fluorescence). Cells were also stained with anti-JAK2 (Cy5, red fluorescence) and Hoechst. (C-D) Analysis of endogenous JAK2 distribution in HEL and K562 cells labeled with Hoechst and anti-JAK2 antibodies. Red lines indicate lines drawn across individual cells in the confocal image, with matching plots below reporting fluorescence intensity. (E) Analysis of nuclear and cytosol extracts from HEL cells, subjected to SDS-PAGE, Western blotting and immunoblotting with JAK2, tubulin, and phospho-histone 3 antibodies.(F-G) Confocal fluorescent images in HEL and K562 cells after transient (HEL) or stable (K562) transfection with JAK2V617F-mCitrine (HEL) or JAK2WT-mCitrine (K562) (green fluorescence). Nuclei were stained with Hoechst and endogenous JAK2 was stained with an anti-JAK2 antibody detected by a secondary antibody coupled to a Cy5 dye. Confocal fluorescent images were acquired using a Zeiss LSM 510 META confocal microscope with a 63× oil objective. ZEN 2009 5.5 SP1 software was used for both image acquisition and processing. Before imaging, treated cells were mounted on slides using ProLong Gold antifade reagent (Invitrogen).
We repeated these experiments in JAK2V617F-positive HEL cells and in JAK2V617F-negative K-562 cells. Again, there was no nuclear localization of endogenous JAK2 in either cell type. Further, JAK2 has a strong plasma membrane localization after transient transfection (HEL), or stable transfection (K562), with the JAK2WT-VFP or JAK2V617F-VFP expression plasmid. Plots below Figure 1C and D provide quantitative analysis of the 2 fluorescence patterns, with no overlap between JAK2 and the nuclear stain. Western blotting experiments following cell fractionation of untransfected HEL cells showed JAK2V617F as a single band, exclusively in the cytosol/membrane fraction, as was tubulin used as a positive control. In contrast, phospho-Histone 3 was found exclusively in the nuclear extract (Figure 1E). These results, which report the distribution of endogenous JAK2V617F, suggest that the addition of VFPs does not alter the cellular location of JAK2 protein.
In summary, our results do not support the conclusions of Rinaldi et al2 and Dawson et al,3 who reported a significant fraction of JAK2V617F to be localized to nuclei of cells. Like Behrmann et al,4 we found JAK2WT, and also JAK2V617F, to be targeted primarily to the plasma membrane and excluded from the nucleus. We note that Rinaldi et al2 also evaluated the cellular localization of JAK2V617F in a group of 10 MPN patients. Nuclear JAK2 was seen in only 3%-5% of the cells from these patients, which corresponded to the fraction of CD34+ cells. We conclude that the bulk of neoplastic cells in MPN are therefore unlikely to rely on nuclear JAK2 for proliferative signals. It remains possible that certain JAK2 variants may uniquely target the nuclei of hematopoietic progenitors, where the functional significance is presently unknown. We suggest that whenever JAK2 or JAK2V617F are detected in the nucleus, the JAK2 gene or/and cDNA be fully sequenced, looking for novel mutations or deletions.
Authorship
Acknowledgments: We thank members of the Wilson and Hermouet laboratories for technical assistance, UMN Cancer Center Microscopy facility, Radek Skoda (University Hospital Basel) for the gift of Baf3/EpoR cells, and Serge Haan (University of Luxembourg) for the gift of the pEf-JAK2-WT-YFP construct. This work was funded by a Leukemia & Lymphoma Society Specialized Center of Research (B.W., project PI). F.G., C.C., and S.H. are members of the MPN&MPNr-EuroNet program.
Contribution: F.G., S.H., and B.W. designed research and wrote the paper; F.G., M.S., and C.C. performed research; and F.G., M.S., C.C., and B.W. analyzed data.
Correspondence: François Girodon, Laboratoire d'Hématologie, Plateau technique de biologie CHU Dijon, 2 rue A Ducoudray, 21070 Dijon cedex. e-mail: francois.girodon@chu-dijon.fr