In this issue of Blood, Lakshmikanthan et al examine the relative roles of the closely related rap1a and rap1b G proteins in angiogenesis.1 Based on previous findings in which angiogenesis was found to be defective in mice with complete knockout of both rap1a and rap1b, they created mice in which rap1 genes were specifically knocked out in endothelium.
They found that the angiogenic defect observed in total rap knockout mice can be recapitulated by an endothelial-specific knockout of rap genes, and that rap1a and rap1b play nonredundant roles in angiogenesis, which rules out the possibility that the angiogenic defect was because of a general loss of an angiotropic factor in tissues that require vascularization.
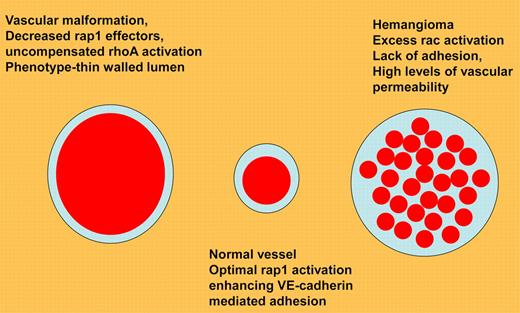
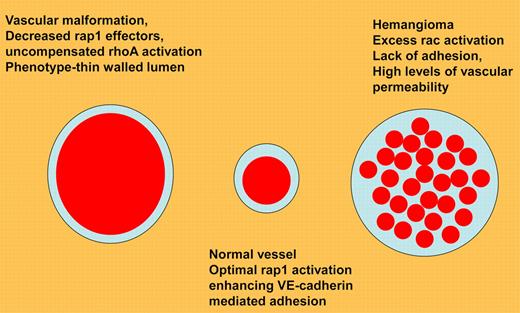
Mulliken and Folkman's classification of vascular anomalies defined by G protein abnormalities.9 Vascular malformation results from lack of rap1 effectors, leading to constitutive rhoA/ROCK activation. Normal vessels have high levels of rap1/integrin/VE-cadherin activation, leading to lack of vascular permeability. Hemangiomas demonstrate constitutive rac activation, and thus can be treated by agents that inhibit rac/reactive oxygen signaling.
Mulliken and Folkman's classification of vascular anomalies defined by G protein abnormalities.9 Vascular malformation results from lack of rap1 effectors, leading to constitutive rhoA/ROCK activation. Normal vessels have high levels of rap1/integrin/VE-cadherin activation, leading to lack of vascular permeability. Hemangiomas demonstrate constitutive rac activation, and thus can be treated by agents that inhibit rac/reactive oxygen signaling.
Rap1b appears to be a stronger mediator of developmental angiogenesis than rap1a. Given these findings, the study by Lakshmikanthan et al focuses on the role of rap1b on VEGFR2 signaling and integrin activation. Rap1b appears to have both integrin β3-dependent and -independent events. Both Rap1b and β3 knockdown appear to reduce the magnitude of VEGFR2 phosphorylation in response to VEGF stimulation. However, the magnitude of stimulation appears to be relatively similar, with the differences in magnitude of final stimulation being dependent on the basal levels of VEGFR2 phosphorylation. This suggests that β3 integrins and rap1b act as rheostats that modify basal levels of VEGFR2 phosphorylation. These results suggest that dual down-regulation of β3 integrin and rap1b would be superior as an antiangiogenic than inhibition of either molecule alone, and suggests that chronic down-regulation of either β3 integrin or rap1b in the clinical setting might lead to a compensatory up-regulation of the other molecule.
Blockade of rap1b has physiologic significance. Down-regulation of rap1b by morpholinos in zebrafish leads to a distinct defect in the number and length of intersomitic vessels of the midtrunk, which is phenocopied in a more severe form by a VEGFR2 kinase inhibitor. This also suggests a developmental gradient, in which midtrunk vessels are more susceptible to rap1b blockade that anterior vessels.
How can we target rap1b? Two studies that should be performed are xenograft studies and carcinogenesis studies. The major question is, how does endothelial rap1b deficiency impact on pathologic angiogenesis? Both xenograft and carcinogenesis studies are suggested because while both of these are examples of pathologic angiogenesis, they have different contributions of local versus bone marrow–derived endothelial contributions. Carcinogenesis experiments are a good measure of local endothelial recruitment, while xenograft studies rely on recruitment of bone marrow precursors, as elegantly demonstrated by the lack of xenograft growth in Id1/Id3 deficient mice.2,3
Finally, abnormalities of rap1 signaling are present in human disease. Loss of Krit1, a rap1 effector, leads to cerebral cavernous malformation, which is associated with uncompensated rhoA activation.4-6 Conversely, hemangiomas are associated with elevated rac1/reactive oxygen signaling (see figure9).7,8 The lessons from the study by Lakshmikanthan et al suggest that rap1 signaling is required for normal vasculature, and that a careful balance of rap1 signaling results in tonic VEGFR2 activation, which allows normal vessels to respond rapidly to an angiogenic stimulus.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health