Abstract
Arsenic trioxide (As2O3) is a highly effective treatment for patients with refractory/relapsed acute promyelocytic leukemia (APL), but resistance to As2O3 has recently been seen. In the present study, we report the findings that 2 of 15 patients with refractory/relapsed APL treated with As2O3 were clinically As2O3 resistant. Leukemia cells from these 2 patients harbored missense mutations in promyelocytic leukemia gene–retinoic acid receptor-α gene (PML-RARA) transcripts, resulting in amino acid substitutions of A216V and L218P in the PML B2 domain. When wild-type or mutated PML-RARA (PR-WT and PR-B/L-mut, respectively) were overexpressed in HeLa cells, immunoblotting showed SUMOylated and/or oligomerized protein bands in PR-WT but not in PR-B/L-mut after As2O3 treatment. Protein-localization analysis indicated that PR-WT in the soluble fraction was transferred to the insoluble fraction after treatment with As2O3, but PR-B/L-mut was stably detected in fractions both with and without As2O3. Immunofluorescent microscopy analysis showed PR-WT localization as a microgranular pattern in the cytoplasm without As2O3 and as a macrogranular pattern with As2O3. PR-B/L-mut was diffusely observed in the cytoplasm with and without As2O3. Nearly identical localization patterns were observed in patients' primary cells. Therefore, B2 domain mutations may play an important role in aberrant molecular responses to As2O3 and may be critical for As2O3 resistance in APL.
Introduction
Acute promyelocytic leukemia (APL) is characterized by the reciprocal chromosomal translocation t(15;17)(q22;q21), leading to fusion of the promyelocytic leukemia gene (PML) on chromosome 15 and the retinoic acid receptor-α gene (RARA) on chromosome 17.1 PML-RARA fusions are detectable in > 95% of patients with APL. In 1985, all-trans retinoic acid (ATRA) was introduced for the treatment of APL as a differentiation therapy, and a dramatic improvement in the overall survival of patients with APL has been obtained.2-4 However, approximately 10%-30% of patients eventually relapse after treatment with combination chemotherapies with ATRA.5-7
Arsenic trioxide (As2O3) is a critical drug for the treatment of APL and is clinically effective even in ATRA-resistant patients.8 As2O3 is a natural substance that has been used medically for over 2400 years. In the 1970s, a group in China identified As2O3 as a component of an anticancer reagent.9 Over the last 18 years, clinical trials conducted worldwide have demonstrated the efficacy of As2O3 for the treatment of relapsed patients with APL.10,11 Recently, it was also reported that As2O3 improves event-free survival and overall survival of adult APL when As2O3 is used as a consolidation treatment after obtaining the first remission.12 Currently, the role of As2O3 in frontline therapy is under investigation.10,13
Rapid degradation of PML-RARA via targeting of PML has been reported as a molecular mechanism for the effectiveness of As2O3.14 Furthermore, As2O3 induces posttranslational modifications of PML-RARA with small ubiquitin-related modifier (SUMO) and ubiquitin, resulting in the transfer of PML-RARA from the soluble fraction to the insoluble nuclear matrix14 and the degradation of both PML and PML-RARA.14-17 In addition to the significant clinical effectiveness of As2O3 for patients with APL, acquired resistance to As2O3 therapy has been recognized in clinical practice.18 Several studies have indicated that arsenic-resistant NB4 cells in vitro show higher glutathione levels than in parental cells.19-21 However, the detailed molecular mechanisms of resistance to As2O3 remain unclear.
Very recently, 2 studies reported that As2O3 binds directly to cysteine residues in zinc fingers located within the RBCC motif that contains 3 cysteine-rich zinc-binding domains, a RING-finger (R), 2 B-box motifs (B1 and B2), and a coiled-coil (CC) domain,22,23 in PML-RARA and PML.24,25 An intriguing hypothesis is that impairment of As2O3 binding to PML-RARA due to conformational changes may result from genetic mutations and/or abnormal posttranslational modifications. These events may be related to resistance to As2O3 therapy.
We report the clinical significance and frequency of As2O3 resistance in patients with APL. Fifteen patients with APL were treated with As2O3 after combination chemotherapy with ATRA, and 2 patients showed clinical As2O3 resistance. Interestingly, in both of these As2O3-resistant patients, missense genetic mutations in the PML-RARA fusion transcript were observed in the leukemia cells. We demonstrated that the mutations, which were located in the PML RBCC region, were critical for PML localization and As2O3 responsiveness in vitro. Our observations suggest that acquired genetic mutations in the PML-RARA transcript may be a critical molecular mechanism of resistance to As2O3 therapy.
Methods
Patients
From January 2000 to December 2008 at Nagoya University Hospital, Japan, 15 patients with APL who showed relapse or disease progression after treatment with chemotherapy with ATRA were treated with As2O3 (Table 1). The diagnosis of APL and its relapse were confirmed by bone marrow morphology according to the FAB classification, chromosomal abnormality t(15;17) in peripheral blood and/or bone marrow cells, positive RT-PCR assay for PML-RARA transcripts, and/or FISH analysis of PML and RARA. As2O3 was diluted in 500 mL of 5% dextrose and administered intravenously over 2 hours at a dose of 0.15 mg/kg daily for a cumulative maximum of 60 days.
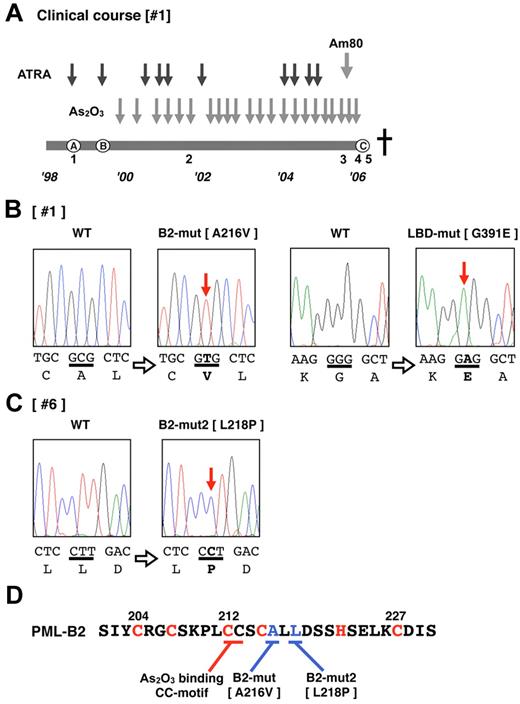
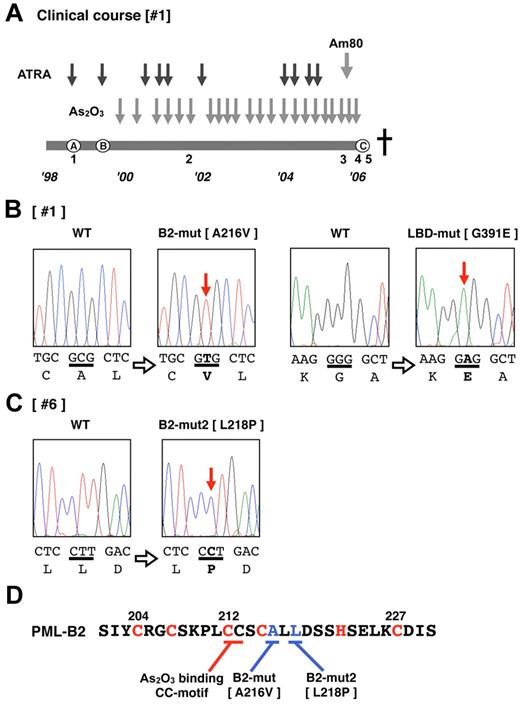
Patient 1 (Table 1 and Figure 1A) was diagnosed with APL (the microgranular variant M3v) in October 1998 (Figure 1A), and complete remission (CR) was obtained after combination chemotherapy with ATRA (45 mg/m2/d). However, relapse with insufficient response to ATRA was observed (Figure 1A) after the end of consolidation therapy in August 1999. As2O3 (0.15 mg/kg/d) was started as a salvage chemotherapy, and a molecular response was obtained. The effectiveness of As2O3 gradually decreased during the patient's 7-year clinical course. Am80 (6 mg/m2/d) was started in July 2005 in addition to As2O3 therapy. However, the effectiveness was poor, and Am80 was discontinued in October 2005. Thereafter, only As2O3 was used. At the terminal stage of his clinical course (Figure 1A), As2O3 was administered under the condition that > 90% of his peripheral blood cells were considered APL blast cells, but no response to the elevated blast count was observed. We diagnosed this condition as secondary As2O3 resistance. The patient died of disease progression in January 2006. During his 7-year clinical course, clinical samples from his bone marrow and/or peripheral blood were obtained repeatedly (Figure 1A).
Patient 6 was diagnosed with APL in January 2000, and CR was obtained after combination chemotherapy with ATRA. Relapse was observed in February 2002. After obtaining a second CR with combination chemotherapy, autologous peripheral blood stem cell transplantation was performed in October 2002. An early relapse was observed after transplantation in February 2003, and then As2O3 (0.15 mg/kg/d) was started. Partial cytoreduction was confirmed, but CR was not obtained. We diagnosed this condition as primary refractory disease to As2O3. As2O3 was used for 42 days and then discontinued. Cord blood transplantation was performed during non-CR, but the patient died of relapsed disease in July 2003. Genomic DNA was obtained from leukemia cells harvested 27 days after starting As2O3 therapy. Although the patient's bone marrow showed hypocellularity at this time, 97.1% of her marrow cells were positive for the PML-RARA fusion gene with FISH analysis.
Cell preparation from patients
After obtaining informed consent from each patient in accordance with the Declaration of Helsinki, the patients' primary cells were obtained from their peripheral blood and/or bone marrow. All experiments were conducted with institutional review board approval from the Nagoya University School of Medicine. Mononuclear cells were separated with Ficoll Paque (GE Healthcare) and preserved with CP-1 (Kyokuto Pharmaceutical Industrial) in liquid nitrogen until further analysis.
RNA extraction and RT-PCR
Total RNA was isolated from each sample using TRIzol (Invitrogen). cDNA was synthesized from 5 μg of total RNA using Superscript II reverse transcriptase (Invitrogen) as described previously.26,27 PCR was performed using LA-Taq polymerase (Takara) under the following conditions: one cycle of 95°C for 4 minutes, followed by 40 cycles of 95°C for 30 seconds, 55°C for 30 seconds, and 72°C for 60 seconds. PCR primers for amplification of the coding sequences of PML-RARA are as follows: forward PR-U619: 5′-TGT TCC AAG CCG CTG T-3′, reverse PR-L2189: 5′-CAT CTT CAG CGT GAT CA-3′. Amplified PCR fragments were purified with a Wizard PCR prep DNA purification kit (Promega) and cloned into the pCR2.1-TOPO cloning vector (Invitrogen). At least 20 clones were sequenced with an ABI 310 automated DNA sequencer (Applied Biosystems). Genetic mutations were confirmed using MacVector Version 10.5.1 software.
Expression vectors
The coding sequence of PML-RARA was amplified with PCR, and the Flag sequence was added with the forward primer as described previously.26,28 The PCR fragment was cloned into the pcDNA4-His-Max-TOPO mammalian expression vector (Invitrogen) to generate the following expression vectors: pcDNA4-XF-PR-WT for Xpress-tagged wild-type PML-RARA, pcDNA4-XF-PR-B/L-mut for Xpress-tagged PML-RARA with mutations resulting in A216V and G391E substitutions, pcDNA4-XF-PR-B2-mut for PML-RARA with the A216V substitution, and pcDNA4-XF-PR-L-B2-mut2 for the long-form PML-RARA with the L218P substitution. To express the long form of the wild-type PML-RARA protein, pcDNA4-XF-PR-L was used as described previously.29 Expression vectors pcDNA-F-Ubc9, pcDNA-F-SUMO, and pcDNA-HA-PML for Flag-tagged Ubc9 and SUMO1, and HA-tagged PML, respectively, have also been described previously.30
Cell culture
Cells of the human cervical cancer cell line HeLa were cultured in DMEM containing 10% FCS. U937 cells, a human monocytic leukemia cell line, were cultured in RPMI containing 10% FCS.
Protein extraction and antibodies for immunoblotting
HeLa cells (1.0 × 105/well) were cultured in a 12-well plate for 12 hours before transfection. Transfection of the expression vectors was carried out using Effectene (Invitrogen) according to the manufacturer's instructions. The cells were washed with DMEM 24 hours after transfection, and then incubated for 8 hours with or without 10μM As2O3 (Sigma-Aldrich) until protein extraction. Whole-cell protein samples for immunoblotting were obtained using 250 μL of Laemmli sample buffer (200mM Tris-HCl, pH 6.8, 4% SDS, 20% glycerol, 10% 2-mercaptoethanol, and 0.004% bromophenol blue). After boiling for 5 minutes, samples were subjected to SDS-PAGE.
U937 cells were cultured in a 12-well plate for 12 hours before transfection. Transfection of the expression vectors pcDNA4-XF-PR-WT and pcDNA4-XF-PR-B/L-mut was carried out using Nucleofector Kit C (Lonza) according to the manufacturer's instructions. After 12 hours, immunofluorescent analysis was performed.
To separate PML-RARA protein into soluble and/or insoluble fractions, cells were lysed in 200 μL of RIPA lysis buffer (50mM Tris-HCl, pH 7.5, 150mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 0.2mM PMSF, and a complete mini protease inhibitor tablet [Roche]). After centrifugation at 10 000g for 10 minutes, the supernatants were placed into new tubes, and 200 μL of 2× SDS sample buffer was added (soluble fraction). PBS (20 μL) and 200 μL of 2× SDS sample buffer were added to the pellets (insoluble fraction). After boiling for 5 minutes, samples were analyzed by SDS-PAGE followed by immunoblotting. Antibodies used in this assay are as follows: rabbit anti-hemagglutinin (anti-HA; Sigma-Aldrich), mouse anti-Xpress tag (Invitrogen), and mouse anti–FLAG-M2 (Sigma-Aldrich).
Immunofluorescence microscopy
HeLa cells expressing Flag- and Xpress-tagged PML-RARA and its mutated proteins were cultured on Chamber Slides (Lab-Tek) with or without 10μM As2O3. U937 cells expressing Flag- and Xpress-tagged PML-RARA and its mutated proteins and the primary leukemia cells were placed on slide glasses using Cytospin (Shandon Southern Products), air dried, and fixed in acetone/methanol for 10 minutes at −20°C. Cells were then blocked with 1% BSA (Sigma-Aldrich) in PBS for 1 hour, incubated with primary antibodies for 3 hours, and incubated with Alexa Fluor 488 (green)- or 568 (red)–conjugated secondary antibodies for 1 hour at room temperature. Antibodies used in this assay are as follows: rabbit anti–human PML (Santa Cruz Biotechnology), mouse anti–FLAG-M2 (Sigma-Aldrich), rabbit Alexa Fluor 488 (Invitrogen), and mouse Alexa Fluor 568 (Invitrogen). The slides were examined with an Axioskop 2 fluorescence microscope (Carl Zeiss), photos were taken and analyzed with AxioVision FRET Release 4.5, and images were processed with Adobe Photoshop CS3 software.
Results
Two of 15 patients showed clinical As2O3 resistance
From January 2000 to December 2008 in Nagoya University Hospital, 15 relapsed patients with APL, including 3 with M3v,31 were treated with As2O3 (Table 1). As a first-line treatment, combination chemotherapies with ATRA were administered to all patients. Thirteen patients received autologous or allogeneic stem cell transplantation after treatment with As2O3, and long-term remission (range, 44-81 months) was confirmed in 10 patients. One patient (patient 1; see also “Patients”) showed resistance to As2O3 after repeated therapy. Another patient (patient 6; see also “Patients”) showed resistance to the first course of As2O3 therapy. Disease progression (elevation of the blast count in the peripheral blood) was observed in patient 1 after long-term treatment with As2O3, and primary refractory disease that was resistant to As2O3 was confirmed in patient 6. Both patients died after disease progression.
Acquired missense mutations in PML-RARA transcripts observed in the 2 patients with As2O3 resistance
To determine the molecular mechanisms of the resistance to As2O3, we first focused on patient 1 who had a long clinical course and whose clinical samples had been preserved several times at each disease stage.
Total RNA was extracted from each sample, and RT-PCR for PML-RARA transcripts was performed. DNA sequencing analysis using the sample obtained from the terminal stage was performed first (Figure 1A sample 5). The PML-RARA transcript was a short-form type (bcr332 ) that lacked the nuclear localizing signal (NLS) in PML. Missense mutations resulting in the A216V substitution in the PML-B2 domain and the G391E substitution in the RARA ligand-binding domain (LBD; Figure 1B) were detected. The predicted mutated PML-RARA protein resulting from these mutations is shown in Figure 2A.
Additional genetic mutations in PML-RARA in patients showing an As2O3 refractory/resistant phenotype. (A) Clinical course of patient 1, who showed As2O3 resistance in the terminal stage of disease progression. The total clinical duration of the patient was almost 7 years. Detailed information is described in “Methods.” Black and gray arrows indicate the course of chemotherapies with ATRA, As2O3, and Am80. The letters A-C enclosed in circles indicate clinically important time points: A indicates diagnosis with APL, B indicates relapse with inadequate response to ATRA, and C indicates confirmation of clinical As2O3 resistance. This patient died of disease progression. Leukemia cells from the bone marrow and peripheral blood were obtained at time periods 1-5. Genetic mutations in PML-RARA were confirmed in patients 1 (B) and 6 (C). Missense point mutations in the PML B2 domain and RARA LBD domain were confirmed in the leukemia cells from time point 5. DNA sequences and the genetic code are indicated in capital letters. Bold letters indicate mutations. (D) The zinc finger motif of the PML-B2 domain. Amino acid substitutions in patients 1 and 6 are depicted. The CC motif critical for As2O3 binding25 is also indicated. The “C” and “H” in red are the cysteine and histidine, respectively, which are important for zinc finger formation.
Additional genetic mutations in PML-RARA in patients showing an As2O3 refractory/resistant phenotype. (A) Clinical course of patient 1, who showed As2O3 resistance in the terminal stage of disease progression. The total clinical duration of the patient was almost 7 years. Detailed information is described in “Methods.” Black and gray arrows indicate the course of chemotherapies with ATRA, As2O3, and Am80. The letters A-C enclosed in circles indicate clinically important time points: A indicates diagnosis with APL, B indicates relapse with inadequate response to ATRA, and C indicates confirmation of clinical As2O3 resistance. This patient died of disease progression. Leukemia cells from the bone marrow and peripheral blood were obtained at time periods 1-5. Genetic mutations in PML-RARA were confirmed in patients 1 (B) and 6 (C). Missense point mutations in the PML B2 domain and RARA LBD domain were confirmed in the leukemia cells from time point 5. DNA sequences and the genetic code are indicated in capital letters. Bold letters indicate mutations. (D) The zinc finger motif of the PML-B2 domain. Amino acid substitutions in patients 1 and 6 are depicted. The CC motif critical for As2O3 binding25 is also indicated. The “C” and “H” in red are the cysteine and histidine, respectively, which are important for zinc finger formation.
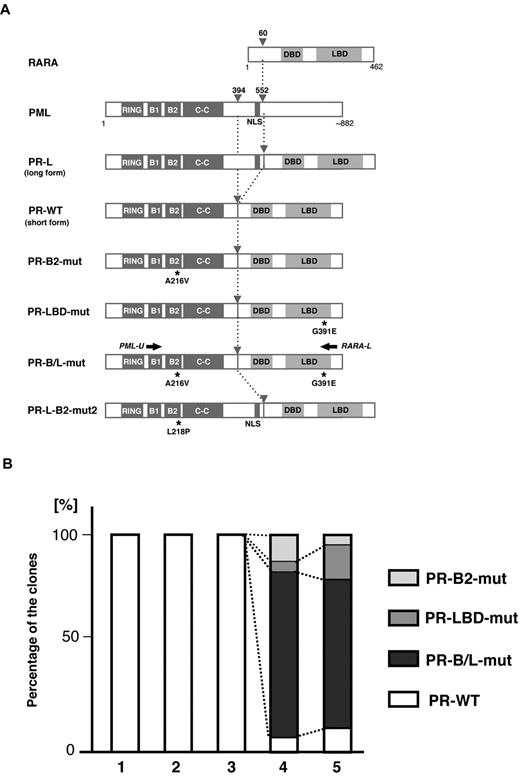
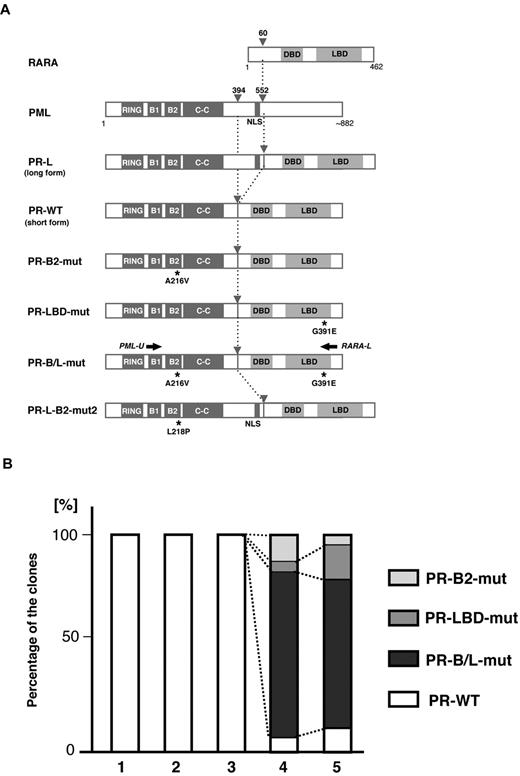
Clonal expansion of PML-B2 and RARA-LBD mutations during disease progression. (A) Schematic representation of PML, RARA, and its fusion proteins with or without mutations. Functional domains are indicated. Gray arrowheads indicate the break points of the fusion proteins. Black asterisks depict amino acid substitutions resulting from genetic mutations. Black arrows indicate the positions of the PCR primers for amplifying PML-RARA fusion transcripts. RING indicates the RING finger; B1 and B2, B-box motifs; C-C, coiled-coil; and DBD, DNA-binding domain. (B) Clonal expansion of PML-RARA mutants. Using the clinical samples obtained at time points 1-5 in Figure 1A, RT-PCR using PCR primers (PML-U and RARA-L in Figure 1A) followed by cloning was performed. At least 20 clones were sequenced for each sample. The percentages of the clones are depicted in the bar graph.
Clonal expansion of PML-B2 and RARA-LBD mutations during disease progression. (A) Schematic representation of PML, RARA, and its fusion proteins with or without mutations. Functional domains are indicated. Gray arrowheads indicate the break points of the fusion proteins. Black asterisks depict amino acid substitutions resulting from genetic mutations. Black arrows indicate the positions of the PCR primers for amplifying PML-RARA fusion transcripts. RING indicates the RING finger; B1 and B2, B-box motifs; C-C, coiled-coil; and DBD, DNA-binding domain. (B) Clonal expansion of PML-RARA mutants. Using the clinical samples obtained at time points 1-5 in Figure 1A, RT-PCR using PCR primers (PML-U and RARA-L in Figure 1A) followed by cloning was performed. At least 20 clones were sequenced for each sample. The percentages of the clones are depicted in the bar graph.
We then determined the sequence of PML-RARA in patient #6, who showed primary refractory disease to As2O3. Only a limited clinical sample obtained at 27 days after starting the As2O3 treatment was available for genomic analysis. A missense mutation resulting in an L218P substitution in the PML-B2 domain was confirmed in 2 of 20 clones with PCR cloning using genomic DNA PCR (Figure 1C). The mutations in the PML-B2 domain are indicated as B2-mut and B2-mut2 in Figure 1D. The predicted mutated PML-RARA protein designated as PR-L-B2-mut2 is also shown in Figure 2A. The location of these mutations is very close to the As2O3-binding cysteine-cysteine (CC) motif reported by Jeanne et al.25
Clonal expansion of PR-B/L-mut at the terminal stage of APL disease progression
To confirm the clonal expansion of the genetic mutations in PML-RARA, we performed sequencing analysis using the RT-PCR fragments of PML-RARA transcripts from the serial clinical samples obtained from patient 1 (samples 1-5 in Figure 1A). PCR fragments were cloned into the vector, and at least 20 clones were picked for sequencing analysis. Genetic mutations resulting in PR-B2-mut, PR-LBD-mut, and PR-B/L-mut (Figure 2A) were confirmed in samples 4 and 5 obtained at this patient's terminal stage when As2O3 resistance and the expansion of the blast count were clinically observed (Figure 2B). These clones were not confirmed in samples 1-3, and the partial response to As2O3 treatment was confirmed at the periods 2-3. The samples 2 and 3 had blasts showing FISH-positive PML-RARA clones (33.9% and 74.0%, respectively). This result strongly suggests that these mutations were closely related to disease progression during As2O3 treatment.
Lack of multimerization of PR-B/L-mut with and without As2O3
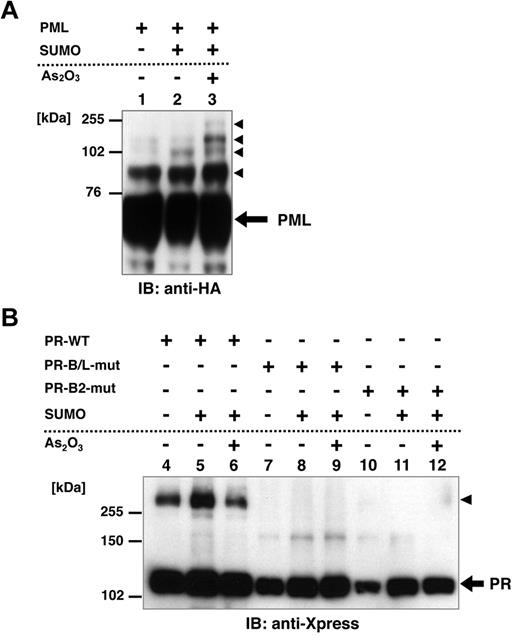
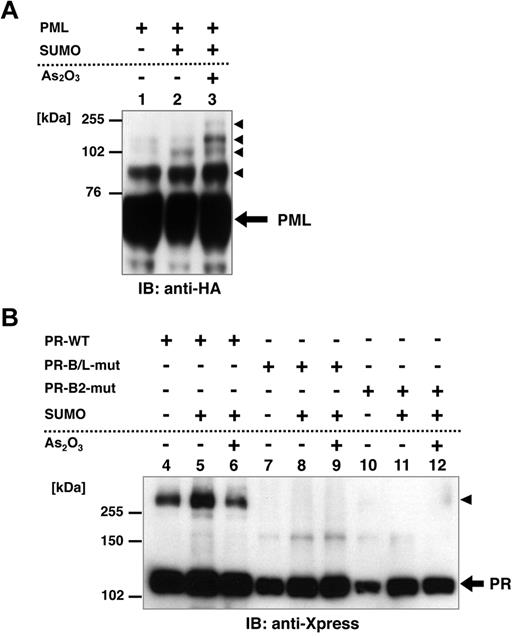
Posttranslational modification of PML, including SUMOylation, is reported to be critical for the responsiveness to As2O3.24,25,33 To confirm the functional difference between PR-WT and its mutant, we performed an in vitro SUMOylation assay in HeLa cells. HA-tagged PML, Xpress-tagged PR-WT, PR-B/L-mut, and SUMO1/Ubc9 were expressed in HeLa cells with or without 10μM As2O3 (Figure 3A-B). PML, PR-WT, and PR-B/L-mut were detected with immunoblotting. SUMO1/Ubc9 is coexpressed with PML, and therefore, SUMOylated PML bands were observed (indicated with black triangles in Figure 3A lane 2). The intensity of the mobility-shifted bands was increased with As2O3 treatment (Figure 3A lane 3). When using PR-WT, multimerized/SUMOylated PR-WT bands were confirmed with and without As2O3 (Figure 3B lanes 4-6 black triangles). However, when using PR-B/L-mut, oligomerized/SUMOylated PML-RARA protein was not observed with SUMO1/Ubc9 with or without As2O3 (Figure 3B lanes 8 and 9). When PR-B2-mut was used in the same assay system, nearly the same result was obtained (Figure 3B lanes 11 and 12). These results indicate that the genetic mutation leading to amino acid alteration of the PML-B2 domain is critical for the appropriate posttranslational modification of the PML-RARA protein, including SUMOylation and oligomerization.
Posttranslational modification of PML, PR-WT, PR-B/L-mut, and PR-B2-mut induced by SUMO1/Ubc9. (A) HA-tagged PML and SUMO1/Ubc9 (SUMO) expression vectors were transfected into HeLa cells as indicated and incubated with or without As2O3 (10μM) for 8 hours. Immunoblot analysis using an anti-HA antibody was carried out. Black arrowheads indicate SUMO-modified PML. (B) Xpress-tagged PR-WT, PR-B/L-mut, or PR-B2-mut was overexpressed in HeLa cells with or without SUMO1/Ubc9 and incubated with or without As2O3 (10μM) for 8 hours. Black arrowhead indicates SUMO-modified/dimerized PR-WT (lanes 4-6). Note that the modified bands of PR-B/L-mut and PR-B2-mut were barely detected (lanes 7-12).
Posttranslational modification of PML, PR-WT, PR-B/L-mut, and PR-B2-mut induced by SUMO1/Ubc9. (A) HA-tagged PML and SUMO1/Ubc9 (SUMO) expression vectors were transfected into HeLa cells as indicated and incubated with or without As2O3 (10μM) for 8 hours. Immunoblot analysis using an anti-HA antibody was carried out. Black arrowheads indicate SUMO-modified PML. (B) Xpress-tagged PR-WT, PR-B/L-mut, or PR-B2-mut was overexpressed in HeLa cells with or without SUMO1/Ubc9 and incubated with or without As2O3 (10μM) for 8 hours. Black arrowhead indicates SUMO-modified/dimerized PR-WT (lanes 4-6). Note that the modified bands of PR-B/L-mut and PR-B2-mut were barely detected (lanes 7-12).
Distinct cellular localization of PR-WT and PR-B/L-mut in soluble/insoluble fractions
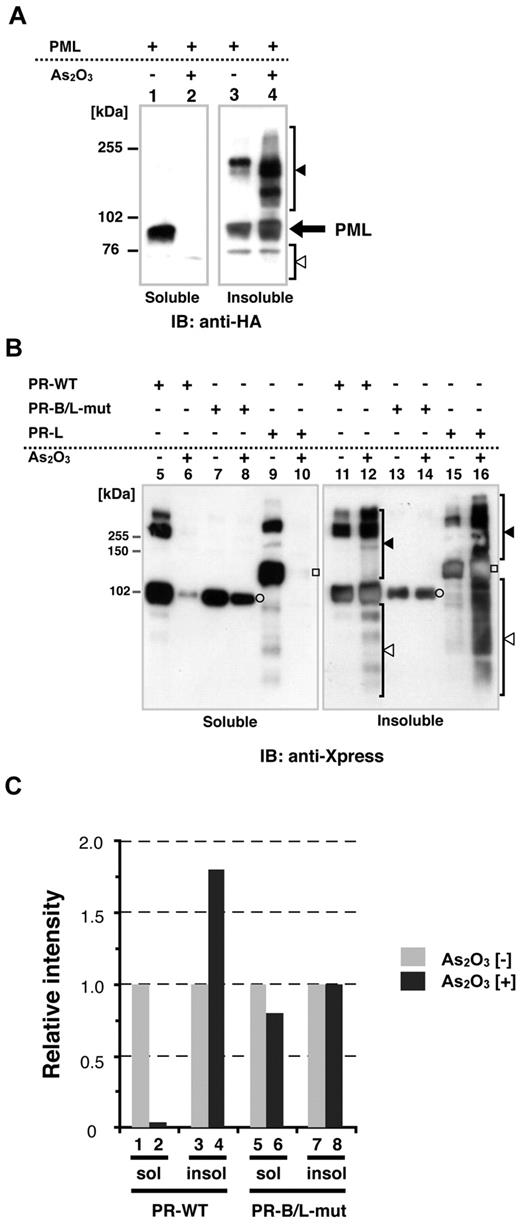
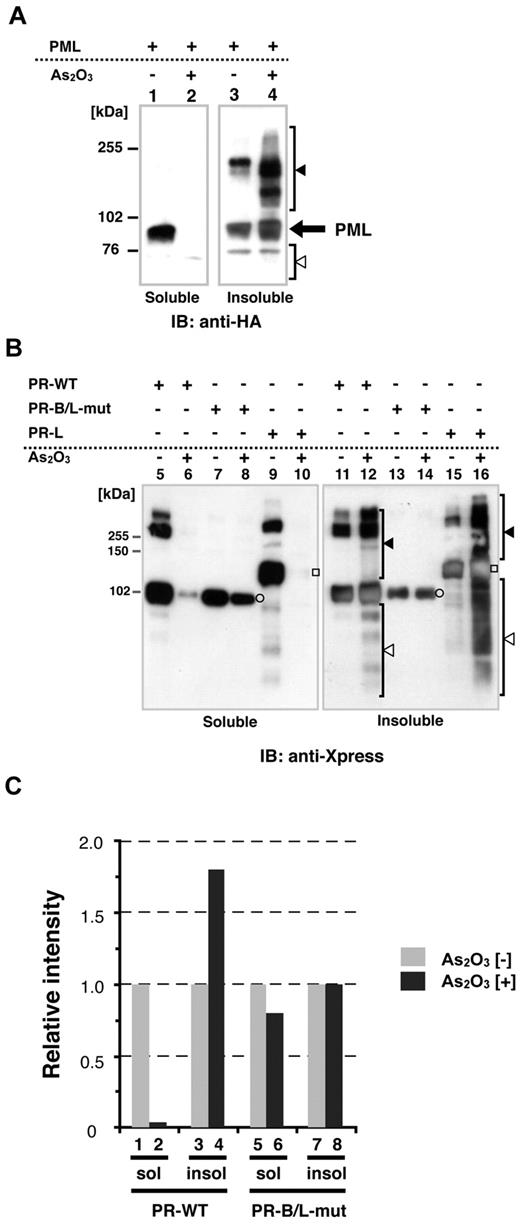
Recently, it has been reported that As2O3 promotes PML-RARA multimerization via disulfide-mediated covalent binding, leading to formation of PML nuclear bodies; these multimers are subsequently SUMOylated.25 To confirm the cellular localization (soluble/insoluble fractions, see also “Methods”) of PML, PR-WT, and PR-B/L-mut with or without As2O3, we performed immunoblotting using HeLa cells that were transiently overexpressing each protein. Cells were lysed using RIPA buffer, as described previously,14,24 and the whole-cell lysate was separated into 2 fractions, soluble (supernatant) and insoluble (pellet). In the absence of As2O3, PML was localized in both the soluble and insoluble fractions (Figure 4A lanes 1 and 3). With As2O3, PML was detected mostly in the insoluble fraction (Figure 4A lanes 2 and 4). Nearly the same results were obtained with PR-WT (short form of PML-RARA, Figure 4B lanes 5, 6, 11, and 12) and PR-long (long form of PML-RARA, Figure 4B lanes 9, 10, 15, and 16). Conversely, PR-B/L-mut was localized in both the soluble and insoluble fractions with and without As2O3, and protein modification including multimerization was not observed with this assay system (Figure 4B lanes 7, 8, 13, and 14). Protein expression levels were quantitated with BioMax software, and the relative intensity is depicted in Figure 4C. These findings indicate that As2O3 did not affect PR-B/L-mut compared with PML and PR-WT in this assay system, and this phenomenon was closely related to insufficient SUMOylation (Figure 3).
Protein localization of PML and its fusion proteins in soluble and/or insoluble fractions with or without As2O3. (A) HA-PML was overexpressed in HeLa cells with or without As2O3 (10μM for 2 hours), and the whole-cell lysate (soluble) and pellets (insoluble fraction) were obtained for immunoblotting. Black and white arrowheads indicate modified/oligomerized and degraded PML, respectively. (B) The same assay described in panel A was performed using PR-WT, PR-B/L-mut, and the long form of PML-RARA (PR-long). White circles indicate PR-WT or PR-B/L-mut and white squares indicate PR-long protein. Black and white arrowheads indicate modified/oligomerized and degraded fusion proteins, respectively. Note that neither protein transportation from the soluble to insoluble fraction (lanes 7 vs 8 and 13 vs 14) nor modified/oligomerized or degraded proteins after treatment with As2O3 (lanes 8 and 14) were observed with PR-B/L-mut. (C) Protein expression levels in panel B were measured using BioMax 1D software, and the relative intensity is depicted as a bar graph.
Protein localization of PML and its fusion proteins in soluble and/or insoluble fractions with or without As2O3. (A) HA-PML was overexpressed in HeLa cells with or without As2O3 (10μM for 2 hours), and the whole-cell lysate (soluble) and pellets (insoluble fraction) were obtained for immunoblotting. Black and white arrowheads indicate modified/oligomerized and degraded PML, respectively. (B) The same assay described in panel A was performed using PR-WT, PR-B/L-mut, and the long form of PML-RARA (PR-long). White circles indicate PR-WT or PR-B/L-mut and white squares indicate PR-long protein. Black and white arrowheads indicate modified/oligomerized and degraded fusion proteins, respectively. Note that neither protein transportation from the soluble to insoluble fraction (lanes 7 vs 8 and 13 vs 14) nor modified/oligomerized or degraded proteins after treatment with As2O3 (lanes 8 and 14) were observed with PR-B/L-mut. (C) Protein expression levels in panel B were measured using BioMax 1D software, and the relative intensity is depicted as a bar graph.
Distinct cellular localization of PR-WT and PR-B/L-mut with or without As2O3 in HeLa and U937 cells
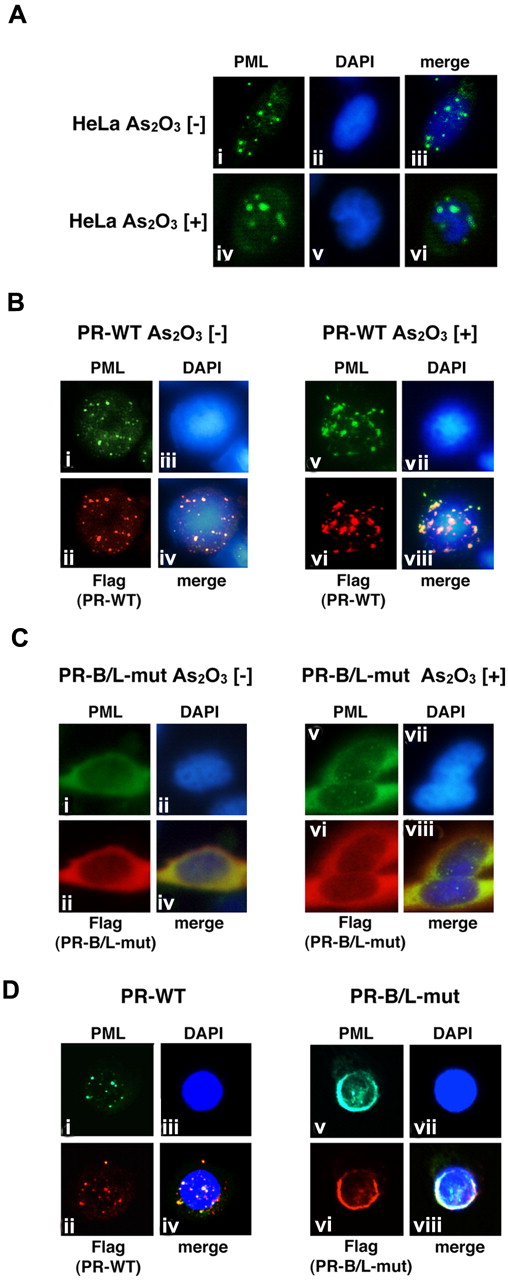
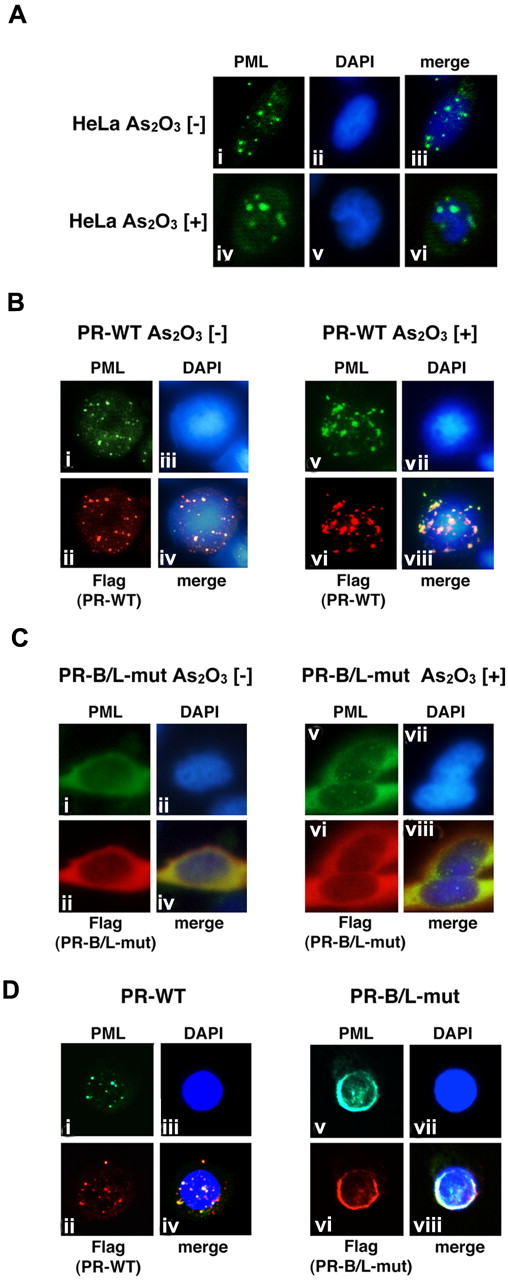
We then performed immunofluorescent (IF) staining to examine the cellular localization pattern of wild-type PML, PR-WT, and PR-B/L-mut in HeLa cells (Figure 5). Wild-type PML was overexpressed in HeLa cells, which were incubated with or without As2O3. PML localization was confirmed by IF staining using an anti-PML antibody. PML was localized in PML nuclear bodies showing a speckled pattern without As2O3 (Figure 5Ai,iii). In the presence of As2O3, the localization pattern was clearly altered to a macrogranular pattern (Figure 5Aiv,vi).
Analyses of the protein localization of PML, PR-WT, and PR-B/L-mut using IF staining. (A) HeLa cells were incubated with or without As2O3 (10μM) for 8 hours, and endogenous PML was detected with an anti-PML antibody. PML nuclear bodies were detected in green. The nuclei were stained with 4,6 diamidino-2-phenylindole (DAPI; blue). Note that the microgranular pattern of NBs without As2O3 (i and iii) was altered to become a macrogranular pattern with As2O3 (iv and vi). (B) Flag-tagged PR-WT was used for the same assay as described in panel A. Anti-FLAG and anti-PML antibodies were used to detect PR-WT and endogenous PML, respectively. PML bodies were confirmed in the cytoplasm with a microgranular pattern without As2O3 (i,ii, and iv) and a macrogranular pattern with As2O3 (v,vi, and viii). (C) When using Flag-tagged PR-B/L-mut, localization showed a diffuse pattern mostly in the cytoplasm with and without As2O3. (D) Flag-tagged PR-WT or PR-B/L-mut was overexpressed in U937 cells without As2O3, and the same IF staining was performed. Note that PR-B/L-mut localization was confirmed in the cytoplasm as a diffuse pattern. Magnification is 800×.
Analyses of the protein localization of PML, PR-WT, and PR-B/L-mut using IF staining. (A) HeLa cells were incubated with or without As2O3 (10μM) for 8 hours, and endogenous PML was detected with an anti-PML antibody. PML nuclear bodies were detected in green. The nuclei were stained with 4,6 diamidino-2-phenylindole (DAPI; blue). Note that the microgranular pattern of NBs without As2O3 (i and iii) was altered to become a macrogranular pattern with As2O3 (iv and vi). (B) Flag-tagged PR-WT was used for the same assay as described in panel A. Anti-FLAG and anti-PML antibodies were used to detect PR-WT and endogenous PML, respectively. PML bodies were confirmed in the cytoplasm with a microgranular pattern without As2O3 (i,ii, and iv) and a macrogranular pattern with As2O3 (v,vi, and viii). (C) When using Flag-tagged PR-B/L-mut, localization showed a diffuse pattern mostly in the cytoplasm with and without As2O3. (D) Flag-tagged PR-WT or PR-B/L-mut was overexpressed in U937 cells without As2O3, and the same IF staining was performed. Note that PR-B/L-mut localization was confirmed in the cytoplasm as a diffuse pattern. Magnification is 800×.
Similar analyses using PR-WT- and PR-B/L-mut–expressing HeLa cells were also performed (Figure 5B-C). Anti-PML antibody was used to detect endogenous PML, overexpressed PR-WT, and PR-B/L-mut, and anti-Flag antibody was used to detect PR-WT and PR-B/L-mut. In the absence of As2O3, PR-WT, a PML-RARA short form without an NLS, was detected around the nucleus as a microgranular pattern (Figure 5Bi,ii,iv), and as a macrogranular pattern in the presence of As2O3 (Figure 5Bv,vi, and viii). Surprisingly, PR-B/L-mut was localized differently in the cytoplasm as a diffuse pattern without As2O3 (Figure 5C,i,ii,iv), and the localization of PR-B/L-mut was not altered in the presence of As2O3 (Figure 5Cv,vi,viii). With As2O3 treatment, endogenous PML was confirmed in the nucleus with the macrogranular pattern (Figure 5Cv,viii). Transfected PR-B/L-mut (red fluorescence) could not be confirmed in the nucleus (Figure 5Cvi). The localization of PR-B2-mut showed almost the same pattern as PR-B/L-mut (data not shown). These data strongly suggest that the point mutation in the PML-B2 domain disrupts or inhibits PML body formation and the responsiveness to As2O3 treatment.
Nearly identical analyses using U937 cells were performed to confirm the significance in hematopoietic cells. As indicated in Figure 5D, PR-WT was observed in the cytoplasm with the microgranular pattern (Figure 5Di,ii,iv), and PR-B/L-mut showed a diffuse pattern, as observed in HeLa cells (Figure 5Dv,vi,viii).
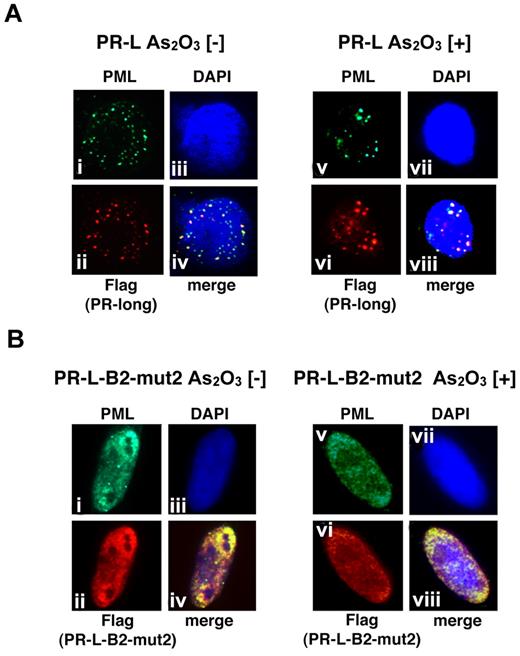
Distinct cellular localization of PR-L and PR-L-B2-mut2 with or without As2O3 in HeLa cells
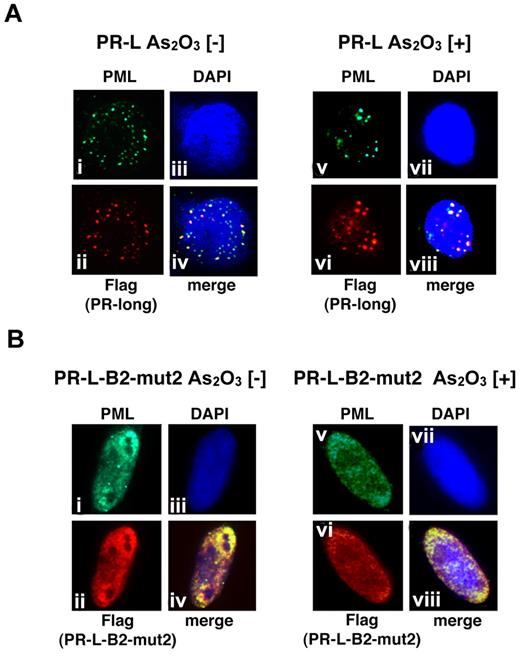
We also performed a similar analysis using PR-L and PR-L-B2-mut2 to show the molecular significance of the L218P mutation in the B2 domain confirmed in patient 6 (Figure 6). PR-L, a PML-RARA long form with an NLS, was detected in the nucleus as a microgranular pattern without As2O3 (Figure 6Ai,ii,iv) and as a macrogranular pattern with As2O3 (Figure 6Av,vi,viii). In contrast, PR-L-B2-mut2 was localized in the nucleus as a diffuse pattern without As2O3 (Figure 6Bi,ii,iv). The localization was not altered in the presence of As2O3 (Figure 6Bv,vi,viii). These data strongly suggest that the L218P mutation in the PML-B2 domain contributes to the aberrant PML body formation and disrupts responsiveness to As2O3 treatment.
Analyses of the protein localization of PR-L and PR-L-B2-mut2 using IF staining. (A) Flag-tagged PR-L was overexpressed in HeLa cells with or without As2O3. Anti-FLAG and PML antibodies were used to detect PR-L and endogenous PML, respectively. PML bodies were confirmed in the nucleus with a microgranular pattern without As2O3 (i,ii, and iv) and a macrogranular pattern with As2O3 (v,vi, and viii). (B) A similar assay was performed using PR-L-B2-mut2. Note that the localization of PR-L-B2-mut2 was confirmed in the nucleus as a diffuse pattern with or without As2O3.
Analyses of the protein localization of PR-L and PR-L-B2-mut2 using IF staining. (A) Flag-tagged PR-L was overexpressed in HeLa cells with or without As2O3. Anti-FLAG and PML antibodies were used to detect PR-L and endogenous PML, respectively. PML bodies were confirmed in the nucleus with a microgranular pattern without As2O3 (i,ii, and iv) and a macrogranular pattern with As2O3 (v,vi, and viii). (B) A similar assay was performed using PR-L-B2-mut2. Note that the localization of PR-L-B2-mut2 was confirmed in the nucleus as a diffuse pattern with or without As2O3.
Cellular localization of endogenous PR-B/L-mut without As2O3 in primary cells from a patient
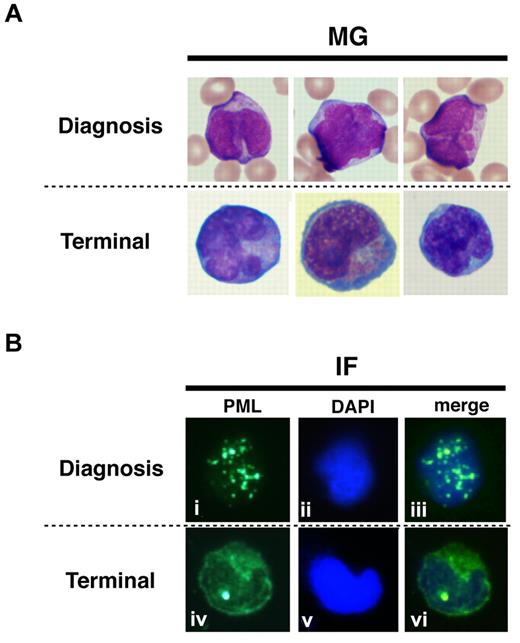
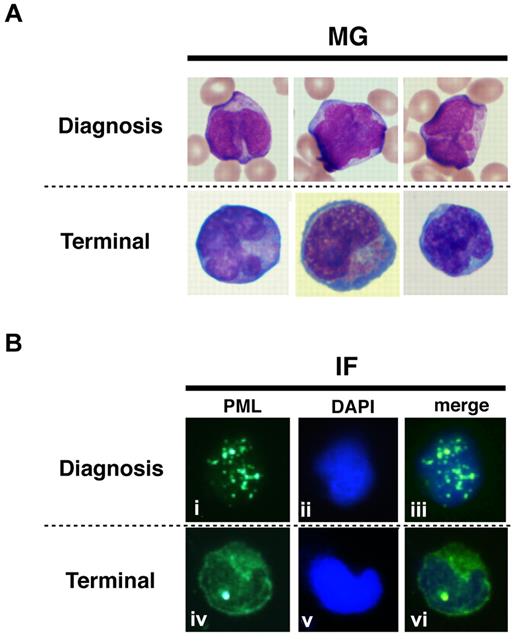
To show the localization of PR-WT and PR-B/L-mut protein in primary leukemia cells, IF staining using an anti-PML antibody was performed (Figure 7). Primary leukemia cells from patient 1 obtained at diagnosis and at the terminal stage were used to detect PR-WT and PR-B/L-mut, respectively. Nearly the same localization pattern as seen in Figure 5 was confirmed in this assay. The PML bodies were observed as a granular pattern at diagnosis (PR-WT), but the pattern was significantly altered to become diffuse at the terminal stage (PR-B/L-mut). These data strongly suggest that PR-B/L-mut protein was expressed and functional in primary leukemia cells, and may contribute to different responsiveness to As2O3 treatment.
Protein localization of PML and its fusion proteins in primary leukemia cells from patient #1. May-Giemsa (MG) staining (A) and IF staining (B) are shown. The primary leukemia cells from patient 1 were obtained at diagnosis and at the terminal stage (shown as 1 and 5, respectively, in Figure 1A) and were used in this assay. PML nuclear bodies can be observed in the cells at diagnosis (i and iii), but at the terminal stage, PML and its fusion proteins were observed in the cytoplasm showing a diffuse pattern (iv and vi).
Protein localization of PML and its fusion proteins in primary leukemia cells from patient #1. May-Giemsa (MG) staining (A) and IF staining (B) are shown. The primary leukemia cells from patient 1 were obtained at diagnosis and at the terminal stage (shown as 1 and 5, respectively, in Figure 1A) and were used in this assay. PML nuclear bodies can be observed in the cells at diagnosis (i and iii), but at the terminal stage, PML and its fusion proteins were observed in the cytoplasm showing a diffuse pattern (iv and vi).
Discussion
In the present study, we have shown acquired missense mutations in PML-RARA that are closely related to resistance to As2O3 treatment. In 2 patients, we detected A216V and L218P substitutions in the B2 domain of PML. The B2 domain is part of the RBCC motif, which is thought to be critical for PML homo/heterodimerization, oligomerization, and As2O3 binding.23-25,34 Recent studies have indicated that As2O3 binds directly to cysteine residues in zinc fingers in the RBCC domain, especially C77/80 and C88/91 in the RING domain24 and C212 and C213 in the B2 domain.25 Binding of As2O3 in the RBCC domain appears to be critical for the effect of As2O3 on PML-RARA.24,25 Interestingly, the mutations described herein were located just adjacent to the CC motif (C212/213) in the B2 domain, which is thought to be critical for As2O3 binding (Figure 1D). These findings suggest that substitutions at A216 and L218 may affect proper As2O3 binding, resulting in aberrant responsiveness to As2O3 through aberrant subcellular localization, insufficient SUMOylation, and/or multimerization.
The in vitro SUMOylation assay indicated that PR-B/L-mut and PR-B2-mut mostly lack SUMO1-Ubc9–induced SUMOylation and dimerization/multimerization (Figure 3B), and this was not changed in the presence of As2O3. It has been suggested that degradation of the PML-RARA protein with As2O3, followed by SUMOylation and oligomerization,24,25 may also be inhibited by mutations in the B2 domain. Furthermore, PR-B/L-mut was localized in both the soluble and insoluble fractions, and the localization was not changed in the presence of As2O3. Nearly the same result was obtained with IF staining, indicating that PR-B/L-mut and PR-B2-mut were localized in the cytoplasm with a diffuse pattern that was not altered in the presence of As2O3 (Figure 5C). Furthermore, PR-L-B2-mut2 was localized in the nucleus as a diffuse pattern with or without As2O3 (Figure 6B). These results strongly suggest that amino acid substitution in the B2 domain is critical for the aberrant responsiveness in vitro, and the mutations may be critical for the resistance to As2O3 therapy in vivo.
In patient 6, only 2 of 20 clones (10%) contained a genetic mutation resulting in an L218P substitution in the B2 domain of the PML region. The genetic mutation was theoretically present in 20% of bone marrow cells, and the remaining 77% of leukemia cells (PML-RARA–positive cells [97.1%] in FISH analysis included PML-RARA–mutated cells [20%]) harbored wild-type PML-RARA according to the FISH analysis. Careful evaluation of whether the mutation contributes to clinical resistance to As2O3 therapy should be performed. Because clinically refractory disease against As2O3 was confirmed after the bone marrow aspiration, clonal expansion of PML-RARA with the L218P substitution may have been related to the insufficient responsiveness to As2O3 therapy. Another question is when the mutated clone appeared in this patient. One possibility is that the mutated clone was already present at the early stage of the disease. Genetic analysis using DNA obtained at the disease onset may be useful for clarifying this. Unfortunately, DNA obtained at the disease onset and the terminal stage of this patient was not available, and clarifying these issues is not currently possible. Further examination of additional patients and genetic analyses of samples obtained at several time points during the disease progression are required.
Interestingly, PR-B/L-mut was localized in the cytoplasm (Figures 5C and 6B), not in the nucleus. As reported previously, an NLS on the PML C-terminus is critical for transport into the nucleus.35,36 The absence of an NLS motif in the short form of PML-RARA (bcr3)32,37,38 may explain the cytoplasmic localization of PR-WT and PR-B/L-mut in our study. Furthermore, PR-B/L-mut and -B2-mut showed a diffuse pattern (Figures 5C-D, 6B, and 7B). Borden et al34 reported that substitution of conserved zinc finger domains in B1 and/or B2 disrupts PML nuclear body formation in vivo, and they also suggested that B1/B2 domains may be involved in the homo/hetero-oligomerization process via protein-protein interactions. Other studies have indicated that PML-RARA with mutations in B2 show a diffuse cytoplasmic pattern, perhaps because the mutated proteins cannot bind to nuclear body–forming proteins such as Daxx, Sp100, and SUMO-1, resulting in a failure to accumulate in the nuclear body.24,25,39,40 Amino acid substitution near the zinc finger motif in the B2 domain may lead to conformational changes in the PML protein. Further analyses are needed.
PR-B/L-mut also contains a mutation in the LBD of RARA (G391E). Previous studies have indicated that the LBD mutation is closely related to ATRA resistance,41-44 and patient 1 in this study also showed a clinically refractory response to ATRA in the late stage of his disease progression and resistance to Am80 in the terminal stage. The LBD mutation G391E was detected only in the terminal stage (Figure 2B lanes 4 and 5). Therefore, an APL variant phenotype with bcr3 of the PML-RARA protein may be related to a poor prognosis,32,37,38 showing that insufficient ATRA effectiveness and the LBD mutation are related to a consistent phenotype of ATRA resistance.45 PR-B/L-mut is localized mainly in the cytoplasm, and the effectiveness of ATRA may not be anticipated. The function of PR-WT and PR-B/L-mut as transcription factors to control activation and/or repression by recruiting coregulators, such as p300/CBP, SMRT/N-CoR-TBL1/R1, and histone deacetylases,26,29,46,47 should be analyzed to confirm the responsiveness to ATRA. Conversely, the probability of the effects of the LBD mutation on As2O3 resistance may be relatively low based on our experiment, because the diffuse localization pattern in the cytoplasm of PR-B2-mut without an LBD mutation in the presence or absence of As2O3 showed nearly the same pattern as PR-B/L-mut (data not shown). Furthermore, a previous study indicated that ATRA-resistant NB4 clones with mutations in the PML-RARA LBD domain were fully sensitive to As2O3 treatment.48
Interestingly, leukemia cells from patient 1 contained minor clones of PR-B2-mut and PR-LBD-mut in addition to the major clone of PR-B/L-mut at the terminal stage (Figure 2B time points 4 and 5), which is difficult to explain. One possibility is that one allele of the wild-type PML and RARA genes originally had genetic mutations in the B2 and LBD domains, and PR-WT, PR-B2-mut, PR-LBD-mut, and PR-B/L-mut were generated at the early stage of disease progression. Another explanation is that the PML-RARA fusion gene with mutations in the B2 and LBD domains translocated again with wild-type PML or RARA genes during the disease progression. Further analyses are required using several strategies including allele-specific PCR, FISH, and/or single nucleotide polymorphism analysis.
Mutations in the B2 domain that result in insufficient responsiveness to As2O3 therapy were confirmed in 2 of 15 (13%) patients with APL treated with As2O3. Recently, As2O3 was introduced as a consolidation treatment, and the event-free survival at 3 years was significantly improved compared with conventional consolidation treatment with ATRA and daunorubicin (80% vs 63%).12 However, almost 5% of patients with APL treated with As2O3 show As2O3 refractory disease.49 Because it is possible that a PML B2 mutation may be partly related to the As2O3 refractory phenotype, repeated genetic analyses at several time points of the clinical course may be useful for predicting patients at high risk for a poor response to As2O3 therapy. Further investigation is required to confirm the clinical significance.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Nobuhiko Emi, Kazuhito Yamamoto, Yukiyasu Ozawa, Taku Kimura, Keiko Niimi, Hiroshi Sao, Kazunori Ohnishi, and Hidehiko Saito for caring for the patients with APL and Tomoka Wakamatsu, Eriko Ushida, Manami Kira, Mari Otsuka, and Yukie Konishi for valuable laboratory assistance.
This work was supported by grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and from the Public Trust Haraguchi Memorial Cancer Research Fund, Japan.
Authorship
Contribution: E.G., A.T., and F.H. designed the experiments, performed the research, and the analyzed data; E.G. and A.T. wrote the manuscript; A.A. prepared the clinical samples and performed the research; and A.T., H.K., and T.N. interpreted the data and supervised the experiments.
Conflict-of-interest disclosure: H.K. is a consultant for Kyowa Hakko Kogyo Co Ltd. The remaining authors declare no competing financial interests.
Correspondence: Akihiro Tomita, Department of Hematology and Oncology, Nagoya University Graduate School of Medicine, Tsurumai-cho 65, Showa-ku, Nagoya 466-8550, Japan; e-mail: atomita@med.nagoya-u.ac.jp.