Abstract
Platelet factor 4 (PF4) and heparin (H) form PF4/H complexes, the target of the immune reaction in heparin-induced thrombocytopenia (HIT). HIT seems to be a secondary immune response as anti-PF4/H-IgG antibodies occur as early as day 4 of heparin treatment. This study investigated whether prevalent infections such as periodontitis may induce the PF4/H immune response as: (1) natural anti-PF4/H Abs are present in the normal population; (2) PF4 bound to bacteria exposes the same antigen(s) as PF4/H complexes; and (3) sepsis induces PF4/H Abs in mice. We found PF4 bound to periodontal pathogens (Aggregatibacter actinomycetemcomitans; Porphyromonas gingivalis) enabling subsequent binding of human anti-PF4/H Abs. The association of natural PF4/H Abs and periodontitis was assessed in a case-control study, enrolling individuals with natural anti-PF4/H Abs (n = 40 matched pairs), and in the cross-sectional population-based Study of Health in Pomerania (SHIP; n = 3500). Both studies showed a robust association between periodontitis and presence of anti-PF4/H Abs independent of inflammation markers (case-control study: lowest vs highest tertile, odds ratio, 7.12 [95% confidence interval, 1.73-46.13; P = .005]; SHIP study, ptrend ≤ 0.001). Thus, preimmunization to PF4/bacteria complexes by prevalent infections, for example, periodontitis, likely explains the presence of natural anti-PF4/heparin Abs and the early occurrence of anti-PF4/H-IgG in HIT.
Introduction
The chemokine platelet factor 4 (PF4; CXCL4) is stored within platelet α-granules1 and released during platelet activation. PF4 binds charge-dependently to polyanions. PF4 has received considerable attention in clinical medicine, as it binds to heparin, thereby forming highly antigenic multimolecular complexes.2,3 The resulting Ab response2 induces the most frequent immune-mediated adverse drug reaction involving human blood cells, heparin-induced thrombocytopenia (HIT).4,5 In HIT, the pathogenic Abs bind to PF4/heparin complexes3 and the resulting immune complexes induce FcR-mediated platelet activation and enhanced thrombin generation. In a subset of patients this causes thrombocytopenia and triggers thrombosis.6
The immune response in HIT is unusual as even patients who receive heparin for the first time generate anti-PF4/heparin-IgG Abs as early as day 4 after start of heparin treatment.7-9 This indicates preimmunization of patients by means other than heparin treatment.
Recently, we have shown that PF4 also binds to polyanions on cell surfaces of aerobic bacteria forming the same Ag as in PF4/heparin complexes.10 Accordingly, mice with polymicrobial sepsis generated anti-PF4/heparin Abs.10 PF4/bacteria complexes are therefore likely candidates for inducing primary immunization which later enables early anti-PF4/heparin-IgG Ab formation during treatment with pharmacologic heparin.
However, sepsis is relatively rare and could not explain why anti-PF4/heparin-IgG Abs are formed in as many as 50% of patients after cardiac surgery within 10 days.11,12 Furthermore, preimmunization by sepsis could not explain the presence of naturally occurring anti-PF4/heparin Abs in 3%-8% of the normal population.6,10,13
We hypothesized that periodontitis might be a cause of preimmunization to PF4/heparin complexes. Periodontitis is one of the most prevalent human infections. The majority of adults suffer from some degree of periodontitis, with 15%-20% of the adult population having severe disease.14 This inflammatory response to the commensal oral flora is primarily provoked by colonization with anaerobic Gram-negative microbes. The resulting inflammation destroys the tissues that support the teeth (periodontium) forming pockets between the gum and tooth.15 This destruction can be measured as probing depth. Probing depth equals the distance from the gingival margin to the pocket base and reflects the current inflammatory status of the periodontal disease. In periodontitis, bacteria can invade the blood stream. Bacteremia occurs more frequently in individuals with periodontal disease than in individuals without periodontitis.16,17 This especially happens during mastication17 or daily oral hygiene measures.18,19 Lockhart et al found bacteremia in 28% of individuals immediately after tooth brushing, and in 79% after tooth extraction.19 It is therefore very likely that bacteremia occurs frequently in individuals with periodontitis.
Periodontitis can also activate platelets by several mechanisms20 and may thereby trigger release of PF4, which can then bind to bacteria forming PF4/bacteria complexes. To assess our hypothesis that periodontal infection may induce a primary immune response to PF4/heparin in humans, we performed in vitro and human studies.
Methods
PF4 binding to periodontal pathogenic bacteria
The major bacterial species associated with periodontitis are Aggregatibacter actinomycetemcomitans (Aa) and Porphyromonas gingivalis (Pg).21 The bacteria used for the PF4 binding study were clinical isolates. The Aa (strain Aa1005Rif, clinical isolate) was kindly provided by Helen C. Schreiner (Department of Oral Pathology and Biology, University of Medicine and Dentistry of New Jersey, Newark, NJ) and Pg (strain ATCC 33277, clinical isolate) was obtained from the DSMZ-German Resource Center for Biologic Material (Braunschweig, Germany). Aa (smooth and rough colonies)22 was grown in AAGM broth consisting of trypticase soy broth (30 g/L) and yeast extract (6 g/L; 0.4% sodium bicarbonate; 0.8% dextrose; 14 hours; 37°C; 5% CO2). Pg was grown in thioglycollate medium enriched with vitamin K1 and hemin (Becton Dickinson) in an anaerobic atmosphere (10% CO2; 37°C; 14 hours; GasPak EZ Pouch System; Becton Dickinson). Bacteria were washed twice with PBS and incubated (30 minutes, 4°C) with biotinylated hu-PF4 (0, 1.25, 2.5, 5, 10, 20, 40 μg/mL). After washing (PBS, 0.05% bovine albumin; 3000g, 5 minutes, 4°C), bacteria were incubated (30 minutes; 4°C) with PerCP-Cy5.5 conjugated streptavidin (BD Biosciences), washed again and fixed (1% paraformaldehyde; 20 minutes, 4°C). Binding of PF4 was analyzed by flow cytometry (Cytomics FC 500; Beckman Coulter). The geometric mean fluorescence intensity (GMFI) multiplied by the percentage of labeled bacteria constituted binding activity.
Ab affinity purification
Aa (smooth and rough form) or Pg were preincubated with PF4 purified from human platelets (20 μg/5 × 107 bacteria, 30 minutes, 4°C; Chromatec) or buffer (PBS, pH 7.4), washed (3000g, 5 minutes, 4°C) and incubated (30 minutes, 4°C) with diluted sera of patients known to contain anti-PF4/heparin-IgG Abs (n = 3 per strain), washed to remove unbound Abs, and bound Abs were eluted with glycine buffer (0.1M, pH 2.7; 5 minutes, room temperature) and the supernatant (eluate) neutralized (Tris buffer; 1M, pH 9). The eluates and untreated sera were tested by PF4/heparin-IgG EIA23 and the heparin-induced platelet activation (HIPA) test.24
Periodontal examinations and definitions
For standardization of periodontal examinations, the dental examiner of the case-control study was calibrated against an experienced examiner from the Study of Health in Pomerania (SHIP).25 In SHIP, calibration exercises were performed parallel to the study each 6-12 months.25 Probing depth was assessed with a periodontal probe (SHIP: PCP-11, case-control study: PCP-2; Hu-Friedy, Chicago, IL). Mean probing depth was based on all assessed periodontal sites. Extent of probing depth was defined as the percentage of sites equaling or exceeding a certain threshold, as indicated for each analysis; for example, probing depth ≥ 3 mm.
Case-control study
Recruitment of cases and controls.
Consecutive blood donors (age 40-60 years) were asked to participate. Exclusion criteria were: < 10 natural teeth; periodontal therapy including dental hygienist treatment within the last 6 months; heparin treatment within the last 3 months. All participants were tested for anti-PF4/heparin Abs of the IgM, IgG, and IgA class by an in-house enzyme-immunoassay (EIA), as previously described.7,23 Sera were tested with freshly thawed samples twice; reproducible ODs > 0.5, inhibited by high heparin (100 IU/mL) ≥ 40%, were considered positive.
EIA results were reported to an independent study coordinator who invited those testing positive to participate in a periodontal examination. Controls were randomly selected among EIA-negative participants but matched for age (± 2 years), sex, smoking status (never, former, current smoking), and school education (< 10, 10, > 10 years). Participants and the dentist were blinded for the PF4/heparin test result.
SHIP study
SHIP is a population-based prospective study conducted in Western Pomerania, Northeast Germany (details25,26 ). A representative sample of 4308 white subjects (age 20-81 years), randomly drawn from population registries, stratified by age and sex, participated. Information on social demographic characteristics, the medical history, and hospital stays during the last 12 months was gathered via computer-aided personal interviews. Sera were tested once (because of limited material) for anti-PF4/heparin IgG, IgA, and IgM Abs, including a high heparin inhibition step. In addition, high-sensitivity C-reactive protein (hs-CRP, Dade Behring Inc; sensitivity 0.2 mg/L), plasma fibrinogen according to Clauss (Electra 1600 analyzer; Instrumentation Laboratory) and leukocyte counts were determined (Coulter MaxM; Coulter Electronics).
Statistical analyses
Statistical analyses were performed with STATA/SE Version 10 (Stata Corp LP). Categorical data are presented as numbers (percentages), and continuous data are given as median (25th; 75th percentiles). A P value < .05 was considered statistically significant.
Laboratory studies.
Differences in reactivities of the eluates were calculated using paired t tests.
Case-control study.
Paired-sample Wilcoxon signed rank tests were used to detect differences in continuous variables between cases and controls. To evaluate the association between periodontal status (exposure) and anti-PF4/heparin Ab status (dependent variable) conditional logistic regression models with fixed effects were used, adjusting for matching variables.27 Odds ratios (OR) for anti-PF4/heparin Abs being associated with periodontal disease were determined by exact methods with median-unbiased estimates (mid-P methods).27 Respective 95% confidence intervals (CI) were reported.
SHIP study.
PF4/heparin EIA ODs were analyzed as continuous variables to assess for any potential association between periodontal status and OD reactivity. Differences in the distribution of variables according to quintiles of mean probing depth were analyzed using Kruskal-Wallis (continuous data) and χ2 tests (categorical data).
The association between periodontal status and anti-PF4/heparin Ab-OD variables was evaluated using linear regression models. Mean probing depth across all measured sites was considered as the main exposure. Analyses were adjusted for age (5-year categories) and sex. Coefficients (B) with their 95% CI were reported. Additional sensitivity analyses were run with the extent of sites presenting a probing depth above a severity threshold as an alternative exposure variable.
The complex sample design was accounted for via survey procedures provided in STATA. Final sampling weights and design variables were used to produce unbiased total estimates.28
Ethics
All subjects gave their written informed consent in accordance with the Declaration of Helsinki. The study protocols were approved by the local ethics committee of Greifswald University.
Results
PF4-binding to periodontal bacteria and of anti-PF4/heparin Abs to PF4-coated periodontal bacteria
PF4 bound in a dose-dependent manner to periodontal bacteria (Figure 1A; Aa smooth colonies > rough colonies > Pg), saturating at 20 μg/mL PF4. Human anti-PF4/heparin Abs bound to PF4-precoated Aa and Pg (Figure 1B). The IgG Abs eluted from PF4-coated bacteria reacted with PF4/heparin complexes in the PF4/heparin-EIA. This shows that PF4 forms the same Ag(s) when bound to bacteria as it forms when bound to heparin. All controls showed the expected results (Figure 1B): (1) the affinity purified Abs did not react with hu-PF4 alone; (2) excess heparin (100 IU/mL), which disrupts PF4/heparin complexes, inhibited anti-PF4/heparin Ab binding; and (3) non-PF4 coated bacteria did not bind the anti-PF4/heparin Abs.
PF4 binds to bacteria found in periodontal lesions and PF4/bacteria complexes are recognized by anti-PF4/heparin IgG from sera of patients with HIT. (A) The anaerobic bacteria A actinomycetemcomitans (smooth and rough colonies; Aa smooth [n = 4] and Aa rough [n = 4]) and P gingivalis (Pg [n = 5]; both clinical isolates from periodontal pockets) were incubated with human biotinylated PF4. All 3 strains bound PF4 with saturation at approximately 20 μg/mL as measured by flow cytometry and expressed as geometric mean fluorescence intensity (GMFI) multiplied with the percentage of labeled bacteria. Data represent mean ± SD of at least 4 independent experiments. (B) From sera of 3 patients with HIT, known to contain anti-PF4/heparin Abs, IgG Abs were affinity purified using hu-PF4–precoated bacteria. Symbols represent reactivities of Abs affinity purified by PF4-coated Aa smooth (■) or rough colonies (▴) and Pg (●); means are presented as horizontal lines. Reactivity of the purified Abs with hu-PF4/heparin complexes (first column) was inhibited by excess heparin (100 IU/mL UFH, column 2), which disrupts PF4/heparin complexes. Abs did not react with hu-PF4 alone (column 3). Non-PF4–coated bacteria served as control for unspecific binding of the Abs to bacteria alone (column 4).
PF4 binds to bacteria found in periodontal lesions and PF4/bacteria complexes are recognized by anti-PF4/heparin IgG from sera of patients with HIT. (A) The anaerobic bacteria A actinomycetemcomitans (smooth and rough colonies; Aa smooth [n = 4] and Aa rough [n = 4]) and P gingivalis (Pg [n = 5]; both clinical isolates from periodontal pockets) were incubated with human biotinylated PF4. All 3 strains bound PF4 with saturation at approximately 20 μg/mL as measured by flow cytometry and expressed as geometric mean fluorescence intensity (GMFI) multiplied with the percentage of labeled bacteria. Data represent mean ± SD of at least 4 independent experiments. (B) From sera of 3 patients with HIT, known to contain anti-PF4/heparin Abs, IgG Abs were affinity purified using hu-PF4–precoated bacteria. Symbols represent reactivities of Abs affinity purified by PF4-coated Aa smooth (■) or rough colonies (▴) and Pg (●); means are presented as horizontal lines. Reactivity of the purified Abs with hu-PF4/heparin complexes (first column) was inhibited by excess heparin (100 IU/mL UFH, column 2), which disrupts PF4/heparin complexes. Abs did not react with hu-PF4 alone (column 3). Non-PF4–coated bacteria served as control for unspecific binding of the Abs to bacteria alone (column 4).
The anti-PF4/heparin Abs affinity purified from PF4-coated Aa (smooth and rough form, 3/3 each) also activated platelets in a functional assay for platelet activating PF4/heparin Abs (HIPA test),24 at low (0.2 IU/mL) but not at high heparin concentrations (100 IU/mL).
Case-control study
General characteristics and anti-PF4/heparin Ab status.
Of 1241 consecutive blood donors, 923 (74.4%) participated. Of those 85 (9.2%) were tested positive; 84 for IgM, one for IgA, and none for IgG. Among those, 40 agreed to undergo periodontal examination. Among 787 negative tested participants, 40 matched controls were recruited (Table 1).
Association between periodontal status and anti-PF4/heparin Ab status.
Anti-PF4/heparin Ab positive individuals presented a worse periodontal status compared with the controls; P < .05 for all probing depth variables. Periodontal disease severity measured by probing depth was significantly related to anti-PF4/heparin-Ab status (Table 2). Subjects within the highest tertile for mean probing depth had a 7-fold higher risk to be tested positive for anti-PF4/heparin Abs compared with the lowest tertile (OR, 7.12 [95% CI, 1.73-46.13]). Extent values for probing depth > 3, 4, and 5 mm presented similar patterns (Table 2).
Study of health in Pomerania
General characteristics.
Among 4308 subjects aged 20-81 years, 3500 (49% men; median age men 52 years, women 49 years) were included in the analysis (missing: 277 anti-PF4/heparin Ab tests; 531 probing depth measurements). Median probing depth was higher in men versus women (2.50 vs 2.27 mm, P < .001), highly correlated with age (r = 0.35, P < .0001), and associated with sociodemographic, behavioral, and laboratory variables (Table 3).
Relationship between probing depth and anti-PF4/heparin Ab status.
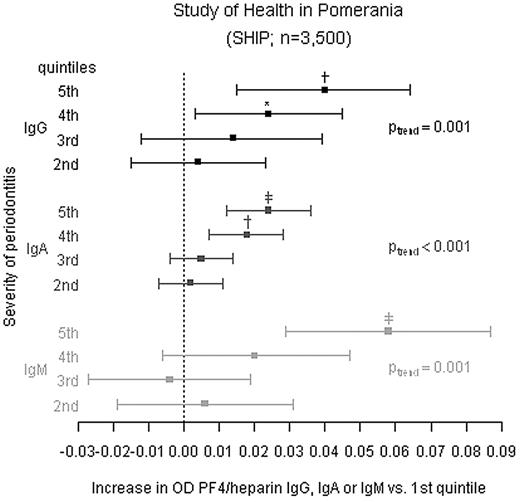
After adjusting for age and sex, mean probing depth was significantly linearly related to the reactivity of the sera in the PF4/heparin EIA as measured by OD (Figure 2, Table 4, M1). On average, mean anti-PF4/heparin OD values increased per quintile compared with the reference quintile for IgG (Ptrend = .001), IgA (Ptrend < .001), and IgM (Ptrend = .001). All findings were consistent if subjects were restricted to those without hospital stays during the last 12 months (Table 4 M2). Models additionally adjusted for inflammatory parameters (quintiles of hs-CRP, leukocytes, and fibrinogen) barely changed estimates across probing depth quintiles (Table 4 M3). Restriction to subjects with anti-PF4/heparin Ab ODs > 0.5 and inhibition of > 40% by high heparin did also not change results markedly (Table 4 M4). Substituting mean probing depth by the extent of probing depth above varying severity thresholds (≥ 3, 4, 5 mm) confirmed the results (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Number of teeth was not associated with the presence of anti-PF4/heparin Abs.
The ODs in the PF4/heparin EIA depend on the periodontal status in non–heparin-treated individuals. In 3500 individuals of the SHIP cohort the periodontal status (assessed by probing depth) and anti-PF4/heparin Ab EIA ODs for IgG, IgA, and IgM were determined. The individuals were grouped into quintiles according to their mean probing depth. Difference in mean ODs in the anti-PF4/heparin EIA (squares) and 95% CIs are given for quintiles 2 to 5. The individuals in the first quintile were used as reference group. Linear regression models were adjusted for age (5-year categories) and gender (reported as M1 in Table 4). Ptrend indicates p for linear trend across quintiles. *P < .05; †P < .01; ‡P < .001 compared with the first quintile. Additional linear regression analyses are given in Table 4.
The ODs in the PF4/heparin EIA depend on the periodontal status in non–heparin-treated individuals. In 3500 individuals of the SHIP cohort the periodontal status (assessed by probing depth) and anti-PF4/heparin Ab EIA ODs for IgG, IgA, and IgM were determined. The individuals were grouped into quintiles according to their mean probing depth. Difference in mean ODs in the anti-PF4/heparin EIA (squares) and 95% CIs are given for quintiles 2 to 5. The individuals in the first quintile were used as reference group. Linear regression models were adjusted for age (5-year categories) and gender (reported as M1 in Table 4). Ptrend indicates p for linear trend across quintiles. *P < .05; †P < .01; ‡P < .001 compared with the first quintile. Additional linear regression analyses are given in Table 4.
Discussion
In this study, we provide several lines of evidence that periodontitis may play a role in the immune response to PF4/heparin complexes. First, PF4 binds to periodontal bacteria. Second, PF4 bound to these bacteria exposes the same Ag as induced by PF4/heparin complexes. This allowed affinity purification of heparin-induced human anti-PF4/heparin Abs using PF4-coated periodontal bacteria. These in vitro findings are substantiated by 2 clinical studies, both showing highly significant associations between the severity of periodontal disease and the presence of natural anti-PF4/heparin Abs. The dose-response relationship between the severity of periodontal disease, which is characterized by an increasing burden of microbes in the periodontal pocket, and the frequency of natural anti-PF4/heparin Abs makes it even more likely that primary immunization to PF4/heparin complexes is induced by bacterial infection(s).
We recently hypothesized that the anti-PF4/heparin Ab response represents an evolutionary ancient host-defense mechanism by which Abs against PF4/heparin complexes can recognize a wide variety of bacteria when this chemokine is bound to them. Moreover, we have shown that phagocytosis of anti-PF4/heparin Ab-coated bacteria is enhanced.10 It would be plausible that such a defense mechanism is present in the mouth were the exposure to bacteria is especially high.
In the ulcerated periodontal pockets, the relatively harmless oral bacteria gain access into the highly vascularized granulation tissue adjacent to the teeth and then into the blood circulation.16,17,29 This everyday occurrence is thought to be one of the key initiators of biologic events which make oral infections an important risk factor for cardiovascular disease, independent of traditional cardiovascular risk factors.30 In fact, common periodontal pathogens have been recovered from carotid endarterectomy plaque samples.31-33 To reach the atherosclerotic plaques, bacteremia must have occurred. Interestingly, PF4 has also been found in these atherosclerotic plaques.34 Whether anti-PF4/heparin Abs interact with leukocytes or platelets in these plaques and potentially even increase local inflammation requires further studies.
One limitation of our study is that we did not quantify the periodontal bacteria either in the pockets nor in the blood. However, the association between subgingival bacterial burden and probing depth is well described in the literature. Demmer et al showed that the severity of periodontal infection (quantified by clinical periodontal measures as in our studies) correlates with bacterial burden, reporting a close correlation (r = 0.61) between the number of sites with probing depth > 3 mm with the subgingival bacterial burden.35 Paju et al reported an association between the number of teeth with deepened pockets and carriage of periodontal pathogens.36
For binding of PF4 and induction of the PF4/heparin immune response, the bacteria have to enter the blood stream. Previous studies already showed that the incidence and intensity of bacteremia correlate with the extent and severity of periodontitis.16,37 It is very difficult to quantify the amount of bacteria that initially gain entrance to the circulation after dental procedures. In volunteer studies, quantification of bacteria in the blood can only be made from venous blood taken from the arm vein. Thus, the blood from the gums has to circulate at least once through the body before it reaches the cubital vein. The periodontal bacteria are diluted by the blood volume and likely most bacteria are already cleared during the first passage in the liver. Furthermore the detection of periodontal Gram-negative anaerobic bacteria is technically very demanding. To overcome the problem of bacterial enumeration and detection Geerts et al quantified the endotoxin load as a proxy for the magnitude of the Gram-negative bacteremia induced by gentle mastication.17 They found endotoxinemia in 6% of individuals before and in 24% after mastication with especially high levels in individuals with severe periodontal disease. Taken together, all these studies unequivocally showed bacteremia occurring regularly in subjects with periodontal disease.
It is well known that anti-PF4/heparin Abs are very transient and disappear within 3 months,7,8 probably because no memory B cells with PF4/heparin specificity are formed.38 We regard this as a very likely reason that the prevalence of anti-PF4/heparin Abs in the general population is not that high. In our opinion the Abs found in the normal population are rather the result from repeated challenges with bacteria which elicits transient production of the anti-PF4/heparin Abs. In this regard, the differences in prevalence of anti-PF4/heparin IgG Abs in the case control study and the cross-sectional population study are of interest. We have previously shown that PF4/heparin Ab formation also depends on patient related factors, such as trauma or inflammation.39 We also speculated before that a special B-cell population, probably marginal zone B cells, might be involved in the immune response of HIT.7 These B cells seem to require a second stimulus, like trauma or inflammation, to produce PF4/heparin Abs, especially of the IgG class. The blood donor population of our case control study had periodontal disease but otherwise they were preselected, healthy regular blood donors. In contrast, the individuals of the cross-sectional population study were also included if comorbidities were present, such as diabetes, poorly controlled hypertension, allergies, bowel disorders, etc. This might be one reason for the low prevalence of anti-PF4/heparin IgG Abs in the blood donor cohort. In line with this hypothesis, we also recently found40 a higher prevalence of anti-PF4/heparin Abs in medical patients at hospital admission and before heparin treatment, compared with blood donors.
We addressed the question whether anti-PF4/heparin Abs are potentially only an epiphenomenon of inflammation and thus an inflammation marker rather than a specific immune reaction. To evaluate this, we included inflammatory markers (hs-CRP, leukocyte counts, and fibrinogen level) known to be increased in periodontal disease41,42 in the regression models (see Table 4 M3). If the association would have been mostly mediated via systemic inflammation, integration of these variables should shift regression coefficients of the exposure (periodontitis assessed by quintiles of mean probing depth) to the Null. However, including inflammatory markers in the multivariate regression model did not attenuate regression coefficients, supporting the assumption that the association was not or only to a minor part mediated by systemic inflammation. This strengthens our hypothesis that the occurrence of natural anti-PF4/heparin Abs may be triggered directly via periodontal pathogens rather than indirectly via systemic inflammation.
Our study has further limitations. The bacteria used for adsorption-elution studies were clinical isolates but not obtained from the immunized individuals themselves and not all these bacteria might have the same binding capacity for PF4. There might also be other, yet unknown, routes of bacterial infection to induce these Abs. We could not assess whether the Abs found in the cross-sectional population study were platelet activating because of lack of material and we do not have follow-up data on whether treatment of periodontal disease lowers the incidence of the anti-PF4/heparin Abs. These questions warrant further studies, given that formation of natural anti-PF4/heparin Abs might represent a potential pathway that could explain the epidemiologic association between cardiovascular morbidity and mortality in subjects with periodontal disease.30
In conclusion, PF4 binds to bacteria typically found in periodontal disease hereby exposing the Ag(s) recognized by human anti-PF4/heparin Abs, and the severity of periodontal disease correlates with the presence of anti-PF4/heparin Abs in otherwise healthy individuals. Preimmunization to PF4/bacteria complexes, caused by prevalent infections such as periodontitis, might therefore explain the presence of natural anti-PF4/heparin Abs and the occurrence of anti-PF4/heparin-IgG Abs as early as day 4 in patients treated with heparin for the first time.8
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
SHIP is part of the Community Medicine Research Net of the University of Greifswald, Germany, which is funded by the Federal Ministry of Education and Research (grant nos. 01ZZ9603, 01ZZ0103, and 01ZZ0403), the Ministry of Cultural Affairs as well as the Social Ministry of the Federal State of Mecklenburg-West Pomerania. D.G. was supported by BMBF-NBL3 program and by Deutsche Gesellschaft für Parodontologie. GABA, Switzerland provided funds to support B.H. K.K. was funded by the Deutsche Forschungsgemeinschaft Graduiertenkolleg GRK-840) and experiments were performed within the Zentrum für Innovationskompetenz Humorale Immunreaktionen bei kardiovaskulären Erkrankungen Federal Ministry of Education and Research FKZ 03Z2CN12, experiments were supported by the Department of Cardiovascular Medicine of the Medical School of the Ernst-Moritz-Arndt University Greifswald (FOKM 2009-08). S.H. was supported by DFG-TRR34, INST 292/110-1.
Authorship
Contribution: A.G. and T.K. designed the study, analyzed and interpreted the data, and wrote the manuscript; K.K., C.W., and S.H. performed the in vitro experiments, designed the bacteria experiments, and wrote the manuscript; B.H. and T.I. designed the statistical part of the study, analyzed the data, and wrote the manuscript; D.G. performed the dental examinations of the case-control study; and all co-authors critically revised the manuscript and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof Dr Andreas Greinacher, Institut für Immunologie und Transfusionsmedizin, Ernst-Moritz-Arndt-Universität Greifswald, Sauerbruchstrasse, D 17475 Greifswald, Germany; e-mail: greinach@uni-greifswald.de.

![Figure 1. PF4 binds to bacteria found in periodontal lesions and PF4/bacteria complexes are recognized by anti-PF4/heparin IgG from sera of patients with HIT. (A) The anaerobic bacteria A actinomycetemcomitans (smooth and rough colonies; Aa smooth [n = 4] and Aa rough [n = 4]) and P gingivalis (Pg [n = 5]; both clinical isolates from periodontal pockets) were incubated with human biotinylated PF4. All 3 strains bound PF4 with saturation at approximately 20 μg/mL as measured by flow cytometry and expressed as geometric mean fluorescence intensity (GMFI) multiplied with the percentage of labeled bacteria. Data represent mean ± SD of at least 4 independent experiments. (B) From sera of 3 patients with HIT, known to contain anti-PF4/heparin Abs, IgG Abs were affinity purified using hu-PF4–precoated bacteria. Symbols represent reactivities of Abs affinity purified by PF4-coated Aa smooth (■) or rough colonies (▴) and Pg (●); means are presented as horizontal lines. Reactivity of the purified Abs with hu-PF4/heparin complexes (first column) was inhibited by excess heparin (100 IU/mL UFH, column 2), which disrupts PF4/heparin complexes. Abs did not react with hu-PF4 alone (column 3). Non-PF4–coated bacteria served as control for unspecific binding of the Abs to bacteria alone (column 4).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/5/10.1182_blood-2011-03-342857/4/m_zh89991175610001.jpeg?Expires=1769779191&Signature=mtvBS-Ur6lEN5LxSNmuxkWweEkkjb0CnPKE7beygf7umrnx7nWYdwWpVFdpplWyTsuEcmbBLUZ2CMNxSIFQN4oGowukfBnLAHseiJEyeKYbGgu5YclWmZbGqqDGXIk2utdBH1TPGWjhpwpPWM8jMD7-QFXIe2NbbUjlpdyZ2aJN5ZrmHI7cDp1uJsqj-~ArdEjiM2evorQTN--As9utcWVBK0j5F9k9XWn6TrWKc1JHXQoPbOQ30tNEYhts9KdWvyPg0S8DnukTsVu6gfDOIrrnTNTpNlCTDZvHJp4N9KfmdCuH0kJL8Fs0F5bjiDLHdA9o5sEi425LLmCEviXf-2Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)